- Submissions

Full Text
Progress in Petrochemical Science
A Mini-Review on Starch-Based Hydrogel and its Application for Drug Delivery of Paracetamol
Nur Shamimi Binti Jaiani, Mohamamd Shahadat*, Mohammad Amir Qureshi and Mohamad Nasir Mohamad Ibrahim
Materials Technology Research Group (MaTRec), School of Chemical Sciences, Universiti Sains Malaysia, 11800 Pulau Pinang, Malaysia
*Corresponding author: Mohamamd Shahadat, Materials Technology Research Group (MaTRec), School of Chemical Sciences, Universiti Sains Malaysia, 11800 Pulau Pinang, Malaysia
Submission: August 27, 2022;Published: November 09, 2022

ISSN 2637-8035Volume4 Issue4
Abstract
Paracetamol is one of the analgesic drugs that will give various side effects if it is taken in high doses. The popularity of hydrogels that release a drug in a temporal, spatial, and dosage-controlled manner has been fueled by recent advancements in polymer chemistry and drug delivery. The present article deals with the synthesis of hydrogel for paracetamol drug delivery. Hydrogels were prepared under mini review different ranges of pH and mixing volume ratios of polymer matrix monomer and crosslinkers as well (e.g., Starch, citric acid, crosslinking, and initiator, etc.). Physical properties like swelling percentage in distilled water and thermal stability have been analyzed to identify quality of prepared hydrogels. In-vitro study revealed the potential of starch-based hydrogels as biodegradable green material for drug delivery. These findings of reported studies revealed that starch-based hydrogels could be effectively employed for the drug delivery of pharmaceutical drug (paracetamol).
Keywords: Starch; Hydrogel; Paracetamol; Drug release
Introduction
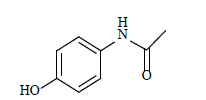
Paracetamol known as acetaminophen is a typical medication that can alleviate pain and reduce fever. Simple analgesic and antipyretic are terms used to describe paracetamol. Contrary to Non-Steroidal Anti-Inflammatory Medicines (NSAIDs), paracetamol has not been shown to reduce tissue inflammation, despite persistent claims that it does so through inhibiting the formation of prostaglandins by the enzyme Cyclooxygenase (COX). Adults and children over the age of 12 should take no more than 1.0g every 4-6 hours, or a maximum of 4.0g per day, of paracetamol [1]. Current therapy procedures rely on the administration of high doses of medications in a systematic manner, which leads to drug resistance and side effects. It is highly desired to have on-demand medication release with a regulated temporal protocol. Drug delivery systems that use the specific area of damaged tissues can be used to target inflammation. The pharmacokinetic and physicochemical features of pharmaceuticals are used to build drug delivery systems [2,3]. The use of appropriate methods and techniques will help in the drug delivery system. Controlled drug release is a superior method of regulating both the frequency of drug delivery and the systematic side effects associated with high drug concentrations. The chemical structure of the paracetamol molecule is shown in Figure 1. There are many techniques to control drug delivery systems. One of them is the solid dispersion technique. By distributing weakly water-soluble pharmaceuticals into water-soluble carriers, the solid dispersion method was originally employed to improve their dissolving characteristics and bioavailability. Despite the incredible promise and substantial research being done on controlled-release solid dispersion systems, commercialization and large-scale production are still a long way off [4]. Microencapsulation technique has remained popular in controlled release due to their relative ease of design and formulation, as well as the benefits of microparticulate delivery methods [5,6]. Encapsulation of the drug into a polymer matrix is one of the most effective approaches for managing drug release systems because it slows the diffusion process.
Figure 1: Chemical structure of paracetamol.
In the present study, we focus on hydrogel due to its highest absorption capacity. Hydrogels are polymer networks that are crosslinked and can absorb a large amount of water [7-9]. It is a soft polymer material having a three-dimensional network structure made up of hydrophilic polymer chains that hold a lot of water without losing their dimensional structure [10,11]. Hydrophilic or hydrophobic functional groups can be found in polymers that create hydrogels. Hydrogels can be made from synthetic or natural polymers, and they can have a variety of chemical compositions as well as mechanical, physical, and chemical properties. The most essential attribute of hydrogels is their biocompatibility, which is described as a material’s ability to come into contact with physiological organs while causing little tissue damage and eliciting unwanted immune reactions. Because of their safety, biocompatibility, hydrophilicity, and biodegradability, natural polymers have recently received increased attention for hydrogel preparation [12,13]. Hydrogels made from biocompatible polymers offer excellent physical qualities like hydrophilicity and elasticity, which aid the controlled release of encapsulated organisms [14,15].
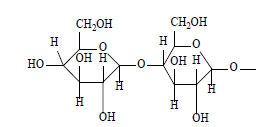
Starch is a common biocompatible polymer used in the production of hydrogels. The most abundant storage polysaccharide in plants is starch, which may be found as granules in the chloroplast of green leaves and the amyloplast of seeds, pulses, and tubers [16]. Amylose and amylopectin are the two primary structural components of starch. It is generally known that starch-based polymers are biodegradable materials, and they have been proposed for application as biomaterials in various fields [17]. Contrarily, hydrophilic acrylic polymers and copolymers comprising a number of acrylamides and acrylic acid have been studied as hydrogels with customizable swelling kinetics. The hydrophilic characteristics of acrylic polymers combined with the biodegradability of starchbased blends provide intriguing hydrogels that may be used as biomaterials. Different attributes are based on the network’s composition and kind of contacts, paying attention to chemical crosslinking and hydrogen bonding interactions. The chemical structure of starch is shown in Figure 2.
Figure 2: Chemical structure of starch.
Starch based hydrogel
Recently, a new class of injectable and biocompatible hydrogel has been developed. Without the use of a conventional chemical crosslinker, N-Succinyl Chitosan (SCS) is combined with watersoluble Dialdehyde Starch (DAS). The -CH=N- group was generated via Schiff’s base reaction between the amine groups of SCS and the dialdehyde groups of DASS, resulting in a hybrid hydrogel. The prepared hydrogels: SCS, DAS, and SCS-DAS hybrid hydrogels were characterized using FTIR analysis to identify functional groups. The findings showed that SCS content has a significant impact on the formation of crosslinked hybrid hydrogels, with an increase in SCS content reducing hydrogel. The properties of the SCS-DAS hybrid hydrogel have the potential to be exploited as a covalent in situ forming a hybrid hydrogel. Tissue engineering and cartilage repair are examples of biomedical applications [18]. In another study, Khaled reported in vitro release of 5-Fluorouracil and Methotrexate from various thermosensitive chitosan hydrogel systems [19]. In this work, multiple thermosensitive chitosan hydrogel systems cross-linked with diverse linking agents, including glycerophosphate, pluronic F127, and hydroxyapatite were loaded with 5-fluorouracil. Methotrexate was also added to 5-fluorouracil to gain synergistic effects. In this study FTIR, Differential Scanning Calorimetry (DSC), and UV-spectrophotometric methods to rule out physical or chemical interactions between the chosen medications and excipients. Then, a modified analytical technique was used to assess the 5-fluorouracil and methotrexate in vitro release characteristics. According to the findings, the cross-linked chitosan hydrogel system containing glycerophosphate and 10% pluronic F127 (F4) had the most acceptable physicochemical characteristics and release profile.
Recently, Jaemin et al. [20] reported the potential of hydrogel for anticancer drug delivery. By combining the self-assembly of 9-fluorenylmethoxycarbonyl-modified diphenylalanine (Fmoc-FF) and its electrostatic interaction with glycol chitosan, a new chitosandipeptide hydrogel was developed (GCS). At a Fmoc-FF mass fraction (FF) of 0.85, the highest gel strength was recorded, and the highest combined strength of the two interactions was attained. The complex hydrogels stability was greatly improved by adding doxorubicin (DOX) as a cationic model medication. Because of the drug’s great gel stability and strong electrostatic attraction binding to Fmoc-FF, DOX-loaded hydrogels displayed sluggish DOX release. Additionally, these hydrogels demonstrated significant thixotropic properties that aided in the creation of injectable self-healing drug delivery systems. Notably, DOX release was markedly accelerated as the medium’s pH dropped from 7.46 to 5.5 and 4.0, probably as a result of the protonation of hydrogel components. Thermosensitive hydrogel for ophthalmic drug delivery was reported by Yu et al. [21]. The hydrogel was developed by the reaction of an aqueous solution of a stimuli-responsive three-dimensional crosslinked hydrogel system made of Carboxymethyl Chitosan (CMC) and poloxamer, a poly (ethylene oxide)/poly (propylene oxide)/poly (ethylene oxide) (PEO-PPO-PEO) block copolymer, underwent a reversible sol-gel transition upon a temperature and/or pH change at a very low concentration. There were more holes obtained in the hydrogels at pH 7.4 and 35 °C than at the other three conditions, which led to more swelling. In this work, nepafenac is used as a model drug and the drug release behavior of these hydrogels was determined.
In addition to that, Jamal et al. [22] reported the anti-cancer potential of the chitosan-based hydrogel. Chitosan and Polyvinyl Alcohol (PVA) were combined to create a chemical hydrogel, which was then crosslinked with triethyl-ortho-formate and loaded with the anticancer medication doxazocin (an alpha-adrenergic blocking agent). The formulation with 8 weights percent crosslinking agent and a doxazocin concentration of approximately 1mg/mL was found the most effective for reducing angiogenesis. El-Hag Ali et al. [23] reported starch-based hydrogel for colon-specific drug delivery systems. In this work, γ-rays were used to induce polymerization and crosslinking of a series of starch/methacrylic acid (MAAc) copolymer hydrogels with various compositions. From the swelling kinetic result, the synthesized hydrogel possessed Fickian diffusion at pH 1 and non-Fickian diffusion at pH 7 recommend as a good aspirant for colon-specific drug delivery. Ren et al. [24] reported thermo-sensitive hydrogel for embedded antibiotics. The authors reported that the CS/gelatine hydrogel prevented the burst effect that was seen with metronidazole. They also showed continuous metronidazole in vitro administration for 12 days. Meanwhile, Qu et al. [25] studied the regulated delivery of amoxicillin from a chemical hydrogel made of chitosan and polyaniline that gelled in place and was responsive to various stimuli, such as pH or voltage. To make the hydrogels conductive and include the medicine before the gelation process, polyaniline was used in this work. Thermo-sensitive chitosan/hyaluronic acid for the pH-sensitive release of doxorubicin was previously studied by Zhang et al. [26]. The authors reported that 30% of doxorubicin was released at pH=4.00 after 120 hours, compared to more than 50% released at pH=6.86 during the same period. In another study, Makarand et al. [27] develop a pH-sensitive controlled drug release method for the delivery of antibiotics using hydrogel based on chitosan and Polyvinyl Pyrrolidone (PVP). The hydrogels were created by creating a semi-permeable polymer network by crosslinking chitosan and PVP blend with glutaraldehyde (semi-IPN). They reported freeze-dried membranes had better drug-release qualities than air-dried hydrogels.
Zhu et al. [28] reported a chitosan-based hydrogel for drug delivery of Kartogenin (KGN). In this report, Glycerol Phosphate (GP) was used to crosslink an injectable hydrogel made of Chitosan (CS) and Hyaluronic Acid (HA). In comparison to hydrogels with lower concentrations of HA, those with higher concentrations had a shorter gelation time, greater water uptake, quicker weight loss, and quicker KGN release. The expression levels of nucleus pulposus markers produced by KGN or TGF- did not significantly differ from one another. Additionally, combining KGN and TGF- did not show a synergistic effect that would have induced nucleus pulposus features. a KGN-conjugated CS/HA hydrogel (5:3:2) with sustained release of KGN in a hydrogel that can encourage ADSC proliferation and nucleus pulposus transformation. Hydrogel for the controlled release of aspirin was reported by Atif et al. [29]. This research sought to create a low-cost, pH-sensitive enteric covering for aspirin using biocompatible polymers. Tetraethoxysilane was used to crosslink solutions of CS carefully and selectively and PVA (5:1 mol ratio). With more crosslinkers, the crosslinking percentage and heat stability improved. The created coating responded to various media, including water, pH (non-buffer and buffer), and ionic media, displaying hydrogel capabilities. They reported in acidic and basic pH conditions, none of the hydrogels swelled much, but at neutral pH, swelling peaked. Commercial aspirin tablets use this hydrogel’s pH sensitivity as an enteric coating. When an enteric-coated aspirin tablet was tested for dissolving in simulated gastric fluid (pH 1.2), 7.11 percent of the aspirin was released within a two-hour period, but 83.25 percent of the remaining aspirin was released over a longer length of time (pH 6.8).
Hydrogel for bovine serum albumin was studied by Li et al. [30]. In this study, they combined N, O-carboxymethyl chitosan with oxidized alginate to create a series of in situ hydrogels without the use of any additional crosslinking agents. They noticed that while gelation was quick at physiological temperatures, it was considerably quicker in the presence of more alginate that had undergone oxidation. After 24 hours of NIH-3T3 cell culture, an in vitro cytotoxicity investigation revealed that the produced hydrogels were not cytotoxic. Additionally, early phases of the release of bovine serum albumin from the hydrogels used diffusion; later stages involved a mechanism that depended on degradation.
Conclusion
Hydrogel is a biocompatible polymer used in drug delivery systems. It can be successfully developed using low-cost biodegradable materials namely starch, citric acid, acrylamide, etc. The results of FTIR have confirmed the existence of the functional groups (-OH, C-H, -C=O, and C-O-) in the matrix of hydrogel that established the formation of starch-based hydrogels. Based on the results of thermal stability of starch-based hydrogels, the created formulation may enable the regulated release of drugs during medical operations. Further study is needed in order to get a better hydrogel for the drug delivery system.
References
- Hayward KL, Powell EE, Irvine KM, Martin JH (2016) Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol 81(2): 210-222.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, et al. (2020) A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv 10: 26777-26791.
- Qureshi MA, Khatoon F (2019) Different types of smart nanogel for targeted delivery. Journal of Science: Advanced Materials and Devices 4(2): 201-212.
- Nehra M, Uthappa UT, Kumar V, Kumar R, Dixit C, et al. (2021) Nanobiotechnology-assisted therapies to manage brain cancer in personalized manner. J Control Release 338: 224-243.
- Arpagaus C (2019) PLA/PLGA nanoparticles prepared by nano spray drying. Journal of Pharmaceutical Investigation 49: 405-426.
- Hickey JW, Santos JL, Williford JM, Mao HQ (2015) Control of polymeric nanoparticle size to improve therapeutic delivery. J Control Release 219: 536-547.
- Ismail H, Irani M, Ahmad Z (2013) Starch-based hydrogels: Present status and applications. Int J Polym Mater Polym Biomater 62(7): 411-420.
- Naeem S, Barkat K, Malik NS, Maryam S (2021) PF-127 based vildagliptin loaded polymeric hydrogels prepared by aqueous polymerization technique for treatment of diabetes mellitus. J Polym Res 28(10): 1-16.
- Qureshi MA, Nishat N, Jadoun S, Ansari MZ (2020) Polysaccharide based superabsorbent hydrogels and their methods of synthesis: A review. Carbohydrate Polymer Technologies and Applications 1: 100014.
- Mahinroosta M, Farsangi ZJ, Allahverdi A, Shakoori Z (2018) Hydrogels as intelligent materials: A brief review of synthesis, properties, and applications. Materials Today Chemistry 8: 42-55.
- Qureshi MA, Khatoon F (2015) In Vitro study of temperature and pH-responsive gentamycin sulphate-loaded chitosan-based hydrogel films for wound dressing applications. Polym Plast Technol Eng 54(6): 573-580.
- Kaczmarek B, Nadolna K, Owczarek (2020) The physical and chemical properties of hydrogels based on natural polymers. Hydrogels Based on Natural Polymers pp. 151-172.
- Qureshi MA, Nishat N, Shahadat M (2022) Industrially and biomedically important guargum based nano composites and their methods of synthesis: A review. Adv Compos Mater pp. 1-23.
- Gull N, Khan SM, Butt OM, Islam A, Shah A, et al. (2020) Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int J Biol Macromol 162: 175-187.
- Qureshi MA, Khatoon F, Rizvi MA, Zafaryab M (2015) Ethyl acetate salix alba leaves extract-loaded chitosan-based hydrogel film for wound dressing applications. J Biomater Sci Polym Ed 26(18): 1452-1464.
- Qamruzzaman M, Ahmed F, Mondal M, Ibrahim H (2021) An overview on starch-based sustainable hydrogels: Potential applications and aspects. J Polym Environ 30(1): 19-50.
- Miculescu F, Maidaniuc A, Voicu SI, Thakur VK, Stan GE, et al. (2017) Progress in hydroxyapatite-starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain Chem Eng 5(10): 8491-8512.
- Kamoun EA (2016) N-succinyl chitosan-dialdehyde starch hybrid hydrogels for biomedical applications. J Adv Res 7(1): 69-77.
- Mohammed AM, Osman SK, Saleh KI, Samy AM (2020) In vitro release of 5-fluorouracil and methotrexate from different thermosensitive chitosan hydrogel systems. AAPS Pharm SciTech 21(4): 1-11.
- Shim J, Kang J, Yun S (2021) Chitosan-dipeptide hydrogels as potential anticancer drug delivery systems. Int J Biol Macromol 187: 399-408.
- Yu S, Zhang X, Tan G, Tian L, Liu D, et al. (2017) A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr Polym 155: 208-217.
- Jamal A, Shahzadi L, Ahtzaz S, Zahid S, Chaudhry AA, et al. (2018) Identification of anti-cancer potential of doxazocin: Loading into chitosan based biodegradable hydrogels for on-site delivery to treat cervical cancer. Mater Sci Eng C Mater Biol Appl 82: 102-109.
- Ali AH, AlArifi A (2009) Characterization and in vitro evaluation of starch based hydrogels as carriers for colon specific drug delivery systems. Carbohydrate Polymers 78(4): 725-730.
- Ren Y, Zhao X, Liang X, Ma PX, Guo B (2017) Injectable hydrogel based on quaternized chitosan, gelatin, and dopamine as localized drug delivery system to treat Parkinson’s disease. Int J Biol Macromol 105(1): 1079-1087.
- Qu J, Zhao X, Ma PX, Guo B (2018) Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized smart drug release. Acta Biomater 72: 55-69.
- Zhang W, Jin X, Li H, Zhang R, Wu C (2018) Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr Polym 186: 82-90.
- Risbud MV, Hardikar AA, Bhat SV, Bhonde RR (2000) pH-sensitive freeze-dried chitosan-polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Control Release 68(1): 23-30.
- Zhu Y, Tan J, Zhu H, Lin G, Yin F, et al. (2017) Development of kartogenin-conjugated chitosan-hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater Sci 5: 784-791.
- Islam A, Yasin T, Bano I, Riaz M (2012) Controlled release of aspirin from pH-sensitive chitosan/poly (vinyl alcohol) hydrogel. J Appl Polym Sci 124(5): 4184-4192.
- Li X, Weng Y, Kong X, Zhang B, Li M, et al. (2012) A covalently crosslinked polysaccharide hydrogel for potential applications in drug delivery and tissue engineering. J Mater Sci Mater Med 23(12): 2857-2865.
© 2022 Mohamamd Shahadat. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






