- Submissions

Full Text
Progress in Petrochemical Science
The Role of Bio-Electrochemical System for Hydrogen Generation
Amit Kumar Chaurasia1* and Lokendra Singh Thakur2
1Department of Chemical Engineering, MVJ College of Engineering, India
2Department of Chemical Engineering, Ujjain Engineering College, India
*Corresponding author:Amit Kumar Chaurasia, Department of Chemical Engineering, MVJ College of Engineering, India
Submission: July 18, 2022;Published: August 02, 2022

ISSN 2637-8035Volume4 Issue3
Abstract
The rapid industrialization and modernisation increase the global energy demands as well as waste generation. The hydrogen production from these waste materials using bio-electrochemical system has shown the capabilities of the commercialized technology. Hydrogen has high energy density and worldwide it is considered as future of the energy sector. The mini review focused on the state-of-the-art technology of Microbial Electrolysis Cell (MEC) that had proven capability of commercialized hydrogen production from almost all the waste materials.
Keywords: Bio-electrochemical system; Microbial electrolysis; Pseudomonas aeruginosa
Introduction
In the last few decades, immense research efforts have been made in the production of hydrogen from waste and renewable resources to fulfil the global energy demand along with reduction in the environmental pollution [1]. Hydrogen (H2) is the clean energy carrier that possess the high energy density and can be utilized in a variety of the applications such as feedstock for industry and refinery [2]. The MEC technologies produce hydrogen gas using the action of exoelectrogens such as Pseudomonas aeruginosa that are capable to degrade the pollutants present in wastewater, which generates e- in the anodic chamber. Further, the e- transfer from bulk of the anode chamber to anode surface takes place through direct and indirect routes with the help of mediators or the microorganisms, which is further transferred to cathode through an external circuit. At cathode surface, the e- diffuses and combines with H+ transferred from anode chamber to form hydrogen gas in presence of an external voltage. As per the literature MECs can concurrently degrade the pollutants present in the wastewater with >60% energy/biohydrogen recovery, however, enhanced biohydrogen recovery requires efficient cathodes or cathode catalysts [3]. The synthesis and development of high electrocatalytic activity cathode requires basic understanding on the working of an MEC and its halfcell reaction mechanism as given in equation 1 to 3.
Anode:
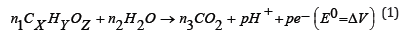
Cathode:
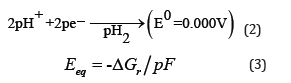
The basic reason behind this is the over-potential of the electrode materials and hydrogen evolution reaction [4]. The development of efficient and economical cathode, operating at ambient temperature and neutral pH is a crucial challenge for Microbial Electrolysis Cell (MEC) to become a commercialized hydrogen production technology (Figure 1). The electrocatalytic activity of the cathode plays a significant role in the proton reduction to hydrogen gas at the surface of cathode by reducing input energy requirement of the MEC. Pt is widely used in MEC as a cathode material; however, its high cost necessitates the development of low-cost cathode material/ catalysts. Electro-deposition of two or more metals on a base metal can create efficient cathode catalysts, which can be competitive to Pt. Presence of phosphate species in cathode material can improve the hydrogen production [5].
Figure 1: Schematic diagram of the Bio-Electrochemical System for Hydrogen Generatio
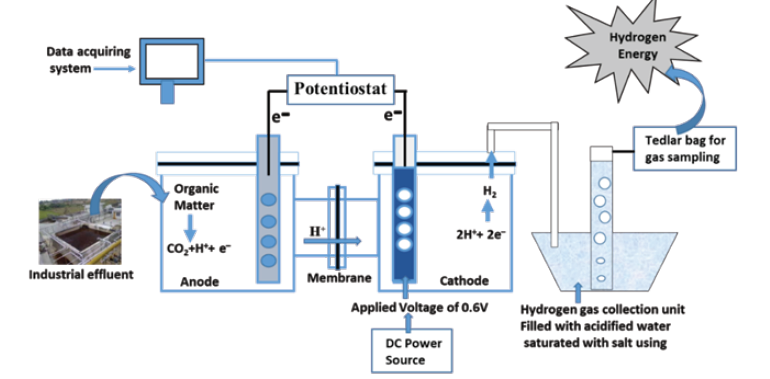
Further, the performance of MEC also depends on the type of wastewater, its composition, characteristics, and presence of impurities in it. Amongst different industrial wastewater, the sugar and paper industry and some other industries wastewaters contain high COD although their composition varies. There are few literatures on the MEC with sugar industry wastewater however, paper industry wastewater has been used rarely in MEC. Further, acetate is considered as an intermediate/end product of anaerobic reactions of organic compounds in water environment and is widely available in various wastewater samples. Apart from these, the cathode catalyst has the great importance in the bio-electrochemical system such as nickel, nickel-cobalt and nickel-cobalt-phosphorous co-deposition on SS and Cu cathodes have shown the good potential to produce low cost and effective cathodes for MEC. The cathode catalyst and types of microorganism have been used to understand the mechanism of hydrogen production, identify rate limit step, role of crystallinity on hydrogen production reactions as well as to determine their corrosion stability and intrinsic hydrogen production capacity [6]. Various MEC performance parameters must be computed and compared to assess their suitability with respect to published literature for industrial application of MEC.
Conclusion
The author has enlisted the key concepts that can be utilized for the commercialized hydrogen production using bio-electrochemical such as the MEC.
References
- Mondal P, Kumari P, Singh J, Verma S, Chaurasia AK, et al. (2017) Sustainable utilization of natural resources. In: Mondal P, Dalai AK (Eds.) (1st Edn), USA, p. 638.
- Chaurasia AK, Mondal P (2022) Enhancing biohydrogen production from sugar industry wastewater using Ni, Ni–Co and Ni–Co–P electrodeposits as cathodes in microbial electrolysis cells. Chemosphere 286(3).
- Chaurasia AK, Shankar R, Mondal P (2021) Effects of nickle, nickle-cobalt and nickle-cobalt-phosphorus nano catalysts for enhancing biohydrogen production in microbial electrolysis cells using paper industry wastewater. J Environ Manage 298: 113542.
- Chaurasia AK, Mondal P (2021) Hydrogen production from waste and renewable resources. Hydrogen Fuel Cell Technology for Stationary Applications, p. 25.
- Chaurasia AK, Goyal H, Mondal P (2020) Hydrogen gas production with Ni, Ni–Co and Ni–Co–P electrodeposits as potential cathode catalyst by microbial electrolysis cells. International Journal of Hydrogen Energy 45(36): 18250-18265.
- Kadier A, Chaurasia AK, Sapuan SM, Ilyas RA, Ma PC, et al. (2020) Essential factors for performance improvement and the implementation of microbial electrolysis cells (MECs). Bio-electrochemical Systems pp: 139-168.
© 2022 Amit Kumar Chaurasia. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






