- Submissions

Full Text
Progress in Petrochemical Science
Graphene Role as a Promotor in Gas Hydrates Formation
Duraisami Dhamodharan, Prabhakar PG and Hun-Soo Byun*
Department of Chemical and Biomolecular Engineering, Chonnam National University, Republic of Korea
*Corresponding author: Hun-Soo Byun, Department of Chemical and Biomolecular Engineering, Chonnam National University, Yeosu, Jeonnam 59626, Republic of Korea
Submission: November 01, 2021;Published: November 15, 2021

ISSN 2637-8035Volume4 Issue3
Abstract
Gas Hydrate (GH) expertise retains excellent ability in environmental and energy fields and accomplishing effective gas hydrate development is crucial for its engineering effort. Graphene (G) is a unique carboncarbon constructed nano assembly substance with exceptional large specific surface area and thermal conductivity. Thus, the application of G-built resources for the advancement of GH development might be viable and has prompted a huge interest. Appropriately, to appraise the modern exploration on G-built application of GH development, this study describes a short review of current studies concerning G-built promoters of GH development. At this time, the research employing numerous kinds of G-built promoters for GH development are recorded and described, the distinctive properties of G-built promoters are reviewed, and the support mechanisms are studied. As a result of this short review, broad insight into G-built promotion of GH development can be achieved, which can lead the layout and uses of innovative G-built developers and might promote to accomplishing effective GH development.
Keywords: Graphene; Gas hydrates formation; Promotor
Abbreviations: GH: Gas Hydrate; CAG: Chemically Altered Graphene; GO: Graphene Oxide; MH: Methane Hydrate
Introduction
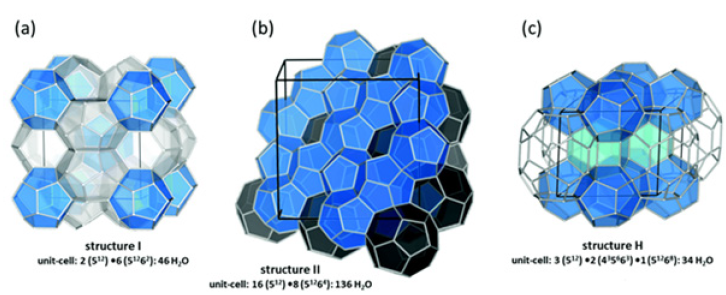
Gas Hydrates (GHs) get trapped an escalating quantity of interest in the early eras due to their outstanding capability for environmental conservation and energy storage [1]. GHs are ice-like crystal clear mixtures constructed by water (H2O) particles (hosts) and gas fragments (guests) in adequate environments. H2O particles form crate-like openings via hydrogen links and trick gas fragments in openings through Van der Waals forces [2]. Generally, built on the quartz constructs of GHs, hydrates are deemed to have followed illustrative categories: Model I, Model II, and Model H (Figure 1), [3]. GHs can attain superior storage volume and are accumulated under moderate circumstances, and consequently are extremely hopeful channels for gas partition, gas storing and shipping, and carbon trap and confiscation [4]. The GH development process encompasses two steps: the seeding stage and the evolution stage. While the seeding stage, the hydrate materials are produced by gas fragments and H2O particles. Though, these hydrate materials are not constant up to they develop to significant shapes, which promotes to a statistical probability and extended seeding time. Subsequently, swift hydrate development is accomplished, and a hydrate layer is firstly produced at the liquid-gas port, which obstructs the transmission of liquid into gas and thus effects in a sluggish hydrate creation rate and minimal H2O to hydrate transformation. The statistical probability initiation period and the minimal development kinetics are the key concerns delaying the engineering utilization of GHs [5]. Consequently, getting effective GH creation is important for the industrial development of GH technology.
Figure 1: Gas hydrate structures (a) Structure I, (b) Structure II, and (c) Structure H. (Reproduced with permission from [3]).
GHs formation is an interfacial prodigy, boosting heat or mass shift in the liquid-gas port can efficiently elevate the hydrate creation kinetic. Mechanical techniques, which consist of gas bubbling, water spraying, stirring, and can accomplish prompt hydrate development through enhancing mass transmission between liquid, and gas. Nevertheless, mechanical techniques require more energy, which causes to high cost and, simultaneously, creates frictional heat in the model, which acts hostile to the heat emitting hydrate creation [6]. For the duration of the early three years, escalating research attention has been given to backers, which play a role as non-mechanical techniques to enrich GH formation. Backers are split into two types: kinetic backers and thermodynamic backers [7]. Kinetic backers enhance mass transfer or heat during GH formation and subsequently rush up the hydrate creation rate [8]. Thermodynamic promoters, enrich hydrate creation through lowering the phase equilibrium environments and facilitating the reaction circumstances [9]. Several surfactants been employed to accelerate the dissolution of gas in H2O by lowering the mass shift resistance and ensued in a superior hydrate creation kinetic and a lessened initiation period. Still, the surfactants engender a substantial sum of froth in the model and protect the water-gas port, which lowers the dissolution of gas in H2O along with triggering diminishes of surfactants [10]. Newly, carbon materials been shown to be effective and economical backers of GHs formation devoid of affecting the frothing difficulty [11]. Diversely, GHs formation is heat emitting process, and the heat engendered during the progression will limit the hydrate materials and damagingly influence hydrate evolution; thus, carbon materials with excellent thermal property can eradicate the excess heat from the model, which upholds the model at a reduced heat and backers the hydrate development highly stable.
Conversely, the carbon materials demonstrate a huge surface area because of their nanoscale structure and dimension, which offers more effective spots for seeding and thus improves mass transmission. Additionally, the non-uniformity of the model will rise in the existence of carbon materials, and heterogeneous seeding will transpire, which constructs hydrate materials very effortlessly than uniform seeding. Hence, the GHs formation can be enhanced by carbon materials [12]. As a new carbon material, graphene (G) shows exceptional thermal conductivity, mechanical strength and expanded surface area, creating it a favorable material for the backers of GHs creation [13]. At this point, we carry out a review targeting on graphene-build (G-build) backers of GHs creation. We primarily discussed the outstanding properties of G-build sources; we later explicate the instances where distinct G-build backers have been employed for GHs creation and debate their backer models.
Role as a Promotor for Gas Hydrate Formation
Graphene (G) and its derivatives
Graphene (G) is a 2D, monolayer thin sheet comprising of honeycomb-assembled and sp2 hybridized carbon-carbon structures [14]. The uncanny layer construction and chemical arrangement bestow G with amazing properties, containing vast surface area, huge transparency, outstanding mechanical properties, and higher electrical and thermal conductivities, which permit G to authorization a broad range of utilization [15]. Neat G is extremely aquaphobic and is impractical to promptly scatter in water devoid of aid or scattering agents, which compels hugescale solution-built fabrication and application developments. G derivatives, for example Chemically Altered Graphene (CAG) and Graphene Oxide (GO), have been made. As compared to neat G, G derivatives hold higher oxygen functionalities or other active spots, which trigger G derivatives to show highly significant and chemical activity and dispersity [14]. G and their related products, further than introduced into various active functional stuffs to form G-build nanocomposites, which could be utilized in the production of sensors, photocatalysis, field-impact transistors, transparent conductive films, green energy devices, etc. [16]. G and G-build nanocomposites are together recognized as G-build materials, all of which have marvelous electrical and thermal properties and exhibiting a nano construction and a vast surface area. Owing to their outstanding properties, G-build stuffs might be extraordinary backers of GH creation: they can competently advance heat transmission by eradicating the heat engendered during GH formation and can in the interim upsurge mass transmission because of their nano construction and hasten nucleation by enriching non-uniformity of the model, which accordingly encourage GH formation. The various studies engaging G-build materials as backers of GH creation are shown in (Table 1).
Table 1: List of some studies engaging G-build materials as backers of gas hydrate creation.
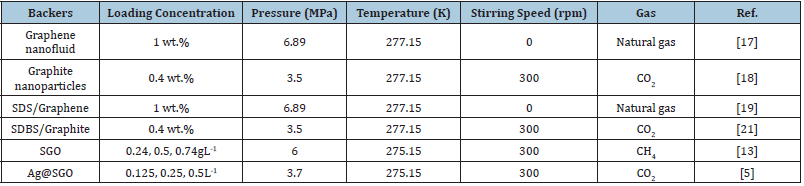
The aquaphobic G has been scattered in H2O to formulate G nanofluid, and it has been utilized as a backer of GH creation [17]. Investigated the impacts of G in natural GH creation and used 1 wt. % of G nanofluid at primary terms of 277.15K and 6.89MPa. The findings revealed that the G nanofluid could lessen the initiation point by 60.06% and improve the storage capability by 13.0% as compared with neat H2O. These enrichments might be belonging to the rise in original liquefied gas in nanofluid, heat transmit coefficient and the heterogeneous nucleation [13]. Utilized G nanofluid to encourage Methane Hydrate (MH) creation at original settings of 277.15K and 6MPa, 300rpm stirring speed and found that G (0.27-0.73g per liter) abridged the hydrate creation time by 46-81% and enhanced the hydrate creation kinetic and the storage capability by 191-661% and 46-71%, correspondingly, as compared with neat H2O. The outcomes shows that the G nano layers not only improved heterogeneous seeding in the model and offered plentiful reactive spots for hydrate seeding but also formed an elevated transfer competence that could confiscate the heat engendered by hydrate creation from the model, subsequently enhancing the efficacy of MH creation. Attributable to comparable promotion procedures, graphite materials also have certain impacts on GH creation [18]. Utilized graphite materials to stimulate carbon dioxide hydrate creation at primary terms of 277.15K and 3.5MPa, 300rpm stirring speed and advised that the initiation point was diminished by 81.0% and the highest carbon di-oxide ingestion was boosted by 13.0% in contrast to neat H2O. They claimed that the elevated heat transfer coefficient and vast surface area of graphite materials engage in crucial roles in fostering carbon di-oxide hydrate creation.
Surfactant-steadied graphene
Also, to the direct promotion of GH creation, G can be blended with surfactants to engender surfactant-steadied nanofluids. G commonly exposes weak scattering and constancy during GH creation, affecting poor execution and cyclability during backer process. Consequently, a combination of G and surfactants been utilized, where the surfactants perform as both co-promoter and stabilizer [19]. Utilized SDS to stabilize G nanofluid (1 wt.%) to formulate SDS/G backer for natural GH creation and shown that the SDS/G backer lowered the initiation period by 19.1% and improved the storage capability by 7.5% as compared to the SDS/H2O model. The decline in initiation period was ascribed to the existence of heterogeneous seeding and an elevated heat transfer coefficient, and the enrichment in storage capability was deemed to be attributable to the boosted gas suspension and heterogeneous reactive spots. Furthermore, the introduction of SDS could enhance the steadiness of nano layers in H2O dissolutions. In addition, fabrication of SDS with reduced graphene oxide and (polyvinylpyrrolidone) PVP, correspondingly, which were employed to support MH creation at primary terms of 273.15K with 4.5MPa. The findings revealed that the fabricated backers both considerably lessened the initiation period and significantly boosted the H2O to hydrate transition while not altering the storage capability [20].
Also, the reduced graphene oxide might create heterogeneous seeding, which shows a lesser active surface energy, triggering shorter free energy and a smaller seeding obstacle than uniform seeding, and is subsequently more kinetically beneficial than homogeneous nucleation. Furthermore, the carbon materials offer various nucleation spots to accelerate seeding. Alternatively, the progress of carbon materials reduced resistance in the liquidgas interface. Thus, the mass transmission was boosted, ahead to a lowered initiation period. A blended graphite material (GM, 0.4 wt.%) distinct loadings of SDBS to make backers and consequently studied the synergistic consequences of SDBS and GM on the rate of carbon di-oxide hydrate creation. The investigational findings revealed that the hydrate storing, gas utilization, water to hydrate conversion and hydrate creation rate were increased by 35.7, 86.5, 20%, and 85.2, respectively, in the existence of SDBS+GM (0.04%) as compared in a neat H2O model. Combining GM into SDBS nanofluid could prevent GM agglomeration and significantly decrease the surface properties of the mixture, getting gas fragments mix in H2O easily, which benefited carbon di-oxide hydrate creation [21].
Graphene carried promotors
Owing to their strong and steady carbon-carbon skeleton, G can act as a nanocarrier to produce new backers of GH creation. Some research work containing the attached sulphonyl groups onto G nano layers via chemical sulfonation to develop an SGO backer, and it act effectively than neat G nanofluid and GO in fostering MH creation. MH formation ended with 0.24-0.74g per L of SGO within 200-300min, and the storage capability achieved 141-151v/v [13]. Conversely, the most of oxygen functional groups, abridged during fabrication, which detached the reticence of H2O pursuit. In contrast, sulphonyl coated G sheets could give a huge interface for methane contents adsorption and H2O particle federation and thus allow to a fast hydrate creation kinetic. Additionally, a new backer called silver decorated SGO been fabricated via attaching silver onto SGO nano layers, and this was consequently utilized as a backer for carbon di-oxide hydrate creation. Ag could offer extra reactive spots for seeding as well-eliminating excess heat from the model and therefore extend accelerated the carbon di-oxide hydrate creation [5]. Moreover, applying G as a nanocarrier for several operational functional groups and many nanoparticles is flexible, an efficient, and viable attempt to making new backers for GH creation and is good-value additional research.
Conclusion and Prospective Points
This study consists of the current investigations on G-build backers of GH creation have been concise, the useful properties and applications of G-build stuffs have been highlighted, and the preferment procedures of G-carried promoters, graphene, surfactant-steadied graphene have been conferred and investigated. G-build stuffs with splendid properties are proficient of populating GH creation: The heat engendered during GH formation can be detached by G-build stuffs owing to their vast thermal property, which upsurges heat transmission in the model and evades the devastation of hydrate materials by elevated temperature; then, G-build stuffs with a huge surface area can enhance mass transmission during GH formation by offering plentiful reactive spots for seeding; in addition, the introduction of G-build stuffs can enrich non-uniformity in the model, and the heterogeneous seeding creates hydrate materials willingly than uniform seeding, efficiently encouraging GH creation. The current research on G-build support of GH creation were executed in lab-made trials, so the support stability, efficient, and reusability of G-build backers in GH creation need to be examined in large scale trials, which is possible in future. Furthermore, further study can emphasis on attaching G/GO with efficient reactive groups to deliver unique G-build promotors or decorating functional nanoparticles onto surfaces of G/GO, targeting to attain new G-build promoters with attractive properties to substantially promote GH creation. Also, due to its nano construction and notable electrical property, G might act as a small size electric rotor that could efficiently mix within small sized restraint areas, which might perhaps be employed for GH support through enhancing mass shift.
References
- Li S, Lv R, Wu Y, Huang F, Zhang X, et al. (2019) Size-aggregation and oxidization dependent perturbation of methane hydrate by graphene nanosheets revealed by molecular dynamics simulations. J Phys Chem C 123(20): 13154-13166.
- Sun Z, Wang R, Ma R, Guo K, Fan S (2003) Natural gas storage in hydrates with the presence of promoters. Energ Convers Manage 44(17): 2733-2742.
- Hassanpouryouzband A, Joonaki E, Farahani MV, Takeya S, Ruppel C, et al. (2020) Gas hydrates in sustainable chemistry. Chemical Society Reviews 49(15): 5225-5309.
- Zhong T, Rogers RE (2000) Surfactant effects on gas hydrate formation. Chem Eng Sci 55(19): 4175-4187.
- He Y, Wang F (2018) Hydrate-based CO2 capture: kinetic improvement via graphene-carried SO3 and Ag nanoparticles. J Mater Chem A 6(45): 22619-22625.
- Fukumoto K, Tobe J, Ohmura R, Mori YH (2001) Hydrate formation using water spraying in a hydrophobic gas: A preliminary study. AIChE J 47(8): 1899-1904.
- He Y, Sun MT, Chen C, Zhang GD, Chao K, et al. (2019) Surfactant-based promotion to gas hydrate formation for energy storage. J Mater Chem A 7(38): 21634-21661.
- Nashed O, Partoon B, Lal B, Sabil KM, Shariff AM (2018) Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J Nat Gas Sci Eng 55: 452-465.
- Lirio CF, Pellegrini PFL, Cohen UAM (2013) Storage capacity of carbon dioxide hydrates in the presence of Sodium Dodecyl Sulfate (SDS) and Tetrahydrofuran (THF). Chem Eng Sci 96: 118-123.
- Veluswamy HP, Hong QW, Linga P (2016) Morphology study of methane hydrate formation and dissociation in the presence of amino acid. Cryst Growth Des 16(10): 5932-5945.
- Park SS, Lee SB, Kim NJ (2010) Effect of multi-walled carbon nanotubes on methane hydrate formation. J Ind Eng Chem 16(4): 551-555.
- Rezaei E, Manteghian M, Tamaddondar M (2016) Kinetic study of ethylene hydrate formation in presence of graphene oxide and sodium dodecyl sulfate. J Petrol Sci Eng 147: 857-863.
- Wang F, Meng HL, Guo G, Luo SJ, Guo RB (2017) Methane hydrate formation promoted by SO3-coated graphene oxide nanosheets. ACS Sustain Chem Eng 5(8): 6597-6604.
- Huang X, Yin Z, Wu S, Qi X, He Q, et al. (2011) Graphene based materials: synthesis, characterization, properties, and applications. Small 7(14): 1876-1902.
- Park S, Ruoff RS (2009) Chemical methods for the production of graphene’s. Nat Nanotechnol 4(4): 217-224.
- Kumar A, Sharma K, Dixit AR (2019) Carbon nanotube- and graphene-reinforced multiphase polymeric composites: review on their properties and applications. J Mater Sci 55: 2682-2724.
- Ghozatloo A, Hosseini M, Shariaty-Niassar M (2015) Improvement and enhancement of natural gas hydrate formation process by Hummers’ graphene. J Nat Gas Sci Eng 27(2): 1229-1233.
- Zhou S, Yu Y, Zhao M, Wang S, Zhang G (2014) Effect of graphite nanoparticles on promoting CO2 hydrate formation. Energ Fuel 28(7): 4694-4698.
- Hosseini M, Ghozatloo A, Shariaty-Niassar M (2015) Effect of CVD graphene on hydrate formation of natural gas. J Nanostruct Chem 5: 219-226.
- Abedi-Farizhendi S, Rahmati-Abkenar M, Manteghian M, Salehzadeh YJ, Zahmatkeshan V (2018) Kinetic study of propane hydrate in the presence of carbon nanostructures and SDS. J Petrol Sci Eng 172: 636-642.
- Yu Y, Xu C, Li X (2018) Evaluation of CO2 hydrate formation from mixture of graphite nanoparticle and sodium dodecyl benzene sulfonate. J Ind Eng Chem 59: 64-69.
© 2021 Hun-Soo Byun. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






