- Submissions

Full Text
Progress in Petrochemical Science
Carbonates the Future of Oil Production
Urdaneta JJ*
Venezuelan Petroleum Corporation (C VP), Venezuela
*Corresponding author: Jhoan Jose Urdaneta, Venezuelan Petroleum Corporation (CVP), Venezuela
Submission: September 10, 2021;Published: October 14, 2021

ISSN 2637-8035Volume4 Issue3
Opinion
Carbonates are the most abundant sediments and sedimentary rocks after terrigenous
clastics. Carbonates are formed mainly by chemical, biochemical and biological processes, in
contrast to sediments and rocks of terrigenous origin, which are essentially originated by the
processes of weathering and erosion of other pre-existing rocks, almost all carbonates are
formed in the marine environment, in coastal or tropical ocean environments where clastic
sedimentation is minimal or does not exist. These carbonates develop as reefs, platforms, atolls,
banks, mounds and ramps, as well as in the form of pelagic deposits in the oceans, for which a
series of conditions are required in the formation and accumulation of these sediments [1]. In
some lake environments with a tropical climate and lakes with high evaporation, carbonates
can also form. Carbonates for their formation, either through direct precipitation or through
organisms when they build their calcareous shells and skeletons, depend on the salinity and
temperature of the waters, on the pH, on the partial pressures of carbon dioxide, dissolved
oxygen, etc. The increase in salinity and temperature, the decrease in carbon dioxide, increase
in oxygen and alkaline pH, favors the precipitation of calcium carbonate.
Carbonate sediments and rocks contain more than 50% carbonate minerals which are
composed of CO3
2- and one or more cations. Calcite (CaCO3) is the most common mineral and
the main component of limestone, followed by dolomite (CaMg(CO3)2). Together these two
minerals form more than 90% of the rock-forming carbonate minerals during geological time.
Limestones and dolomites can contain varying amounts of quartz, feldspars, and clay minerals.
In smaller quantities and locally, antigenic minerals such as chert, gypsum, anhydrite and
pyrite can be found. In recent carbonates the common minerals are calcite and aragonite.
Calcite splits into high Mg calcite (>5% MgCO3) and low Mg calcite (<5% MgCO3). Aragonite
and calcite with high Mg are metastable and will invariably change to the stable form that is
calcite with low Mg. These minerals are formed biochemically by certain organisms, or by
inorganic precipitation forming cements or constituents such as ooids and in the same way it
happened in the geological past [2]. However, due to their condition as metastable minerals,
diagenetic changes will operate on aragonite and calcite high in Mg, adapting a form of greater
stability. Therefore all fossils with shells or skeletal parts, inorganic constituents and cements
of these metastable minerals prior to the middle part of the Middle Pleistocene, will change to
the stable form that is calcite with low Mg or simply calcite. In modern carbonate sedimentary
environments, dolomite is not as common as in the past since this mineral is formed basically
by dolomitization processes from calcite, high magnesium calcite and aragonite. However,
current models in which dolomitization processes are being developed, such as hypersaline
lagoon areas, sabkhas mixing zones of meteoric and marine waters, among others, have made
it possible to understand the processes and formation of dolomites [3].
Diagenetic changes profoundly alter the sediments and organisms formed by CaCO3.
Most of these changes occur on the surface or by processes derived from it, during the early
stages of burial. By modifying the initial mineralogy, the processes of lithification, formation
of secondary cements and transformation or creation of porosity manifest from the initial
moment of deposition (Figure 1). Carbonate minerals are found in numerous sedimentary
environments, some terrestrial, but it is in tropical marine environments where they
present a great abundance, both in the present and in the geological past, representing an
excellent paleoclimatic indicator. Carbonate minerals are formed from carbonate-saturated
waters by biochemical processes developing the skeletal parts and shells of calcareous organisms, as well as by chemical precipitation of supersaturated
waters, forming concentric or radial laminations (ooids), and in
environments with high evaporation or in the walls of caves and
caverns due to rapid reduction of CO2 (stalagtites and stalagmites)
[4]. Limestones and dolomites represent between 1/5 to 1/6 of
the global sediments and sedimentary rocks and from these it is
possible to know, more than from any other group of sedimentary
rocks, about the geological evolution of the Earth’s surface.
The sedimentary particles and the depositional texture of the
limestones are indicative of environments, facies, current energy,
erosion factors, etc. The limestone fossils represent the evolution
of organisms, palaeoenvironments, paleoclimates and changes in
sea level, from the precambrian to the present day. The chemical
composition of limestones and dolomites and the content of the
fossils indicate the physicochemical characteristics of the fluids and
the environment and the conditions of temperature, salinity, water
depth, oxygenation, etc.
Figure 1: Division of the cut with the results of the discretization of porosity in a well in western Venezuela.
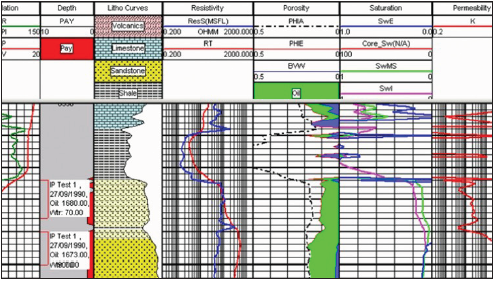
Carbonate classifications present a higher degree of complexity than those used for clastic sediments such as sandstones. More than the textural characteristics or chemical composition, the depositional texture, genesis and recognition of the constituents, are necessary for a good classification. The wide variety of porosities, their origin and modification, as well as their relationship with the initial depositional environments and underground stages, create an inexhaustible topic of discussion in carbonates. By means of isotopic measurements with oxygen used by calcareous organisms, mainly some species of planktonic foraminifera, paleotemperature values and glaciation and interglaciation states are obtained. In this way, curves of sea level changes have been developed, mainly during the Tertiary and Quaternary periods. Limestones and dolomites generally constitute aquifers and hydrocarbon deposits, as well as deposits of zinc, lead, silver and mercury [5]. As important hydrocarbon reservoirs, they represent approximately 50% of the world’s deposits. In the deposits of the Persian Gulf and in Mexico, most of the hydrocarbon deposits are stored in limestone and dolomites. There are also important reservoirs in the lower cretaceous in some regions of the United States (primarily Texas), as well as in various calcareous paleozoic facies in Canada and the United States. Hydrocarbon deposits in carbonates are found in North Africa, mainly Libya and Algeria, as well as in southern Russia. In Venezuela most of the deposits correspond to sandstone facies, but important limestone and dolomite deposits are found in the lower cretaceous of the Lake Maracaibo basin, in front of Perijá and areas of tidal plains of the Barinas region (Cenomanian). Some limestones can be used in the chemical industry as a source of CaO, as well as for cement production and use in the construction industry.
References
- Braga JC, Martín JM, Puga Bernabéu A (2015) Origin of porosity and permeability in sediments and carbonate rocks. pp. 1-71.
- Pérez RO, Grijalva N, Francisco J, Montijo G, Alejandra (2012) Environments of carbonated sedimentation. Carbonate rocks, University of Sonora, Mexico.
- PDVSA (2002) Manual of operating procedures for carbon rocks.
- Walker M (1992) Facies models response to sea level change. In: Walker RG, James NP (Eds.), Geological Association of Canada, UK, 29(3): 291-291.
- Singer JM (1997) WEC: Venezuela Well Evaluation Conference. Schlumberger, USA.
© 2021 Jhoan Jose Urdaneta. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






