- Submissions

Full Text
Progress in Petrochemical Science
The Future of Carbon Dioxide
Ryczkowski J*
Department of Chemical Technology, Poland
*Corresponding author: Ryczkowski J, Department of Chemical Technology, Poland
Submission: January 21, 2021;Published: January 29, 2021

ISSN 2637-8035Volume4 Issue1
Introduction
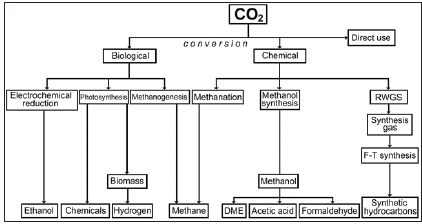
Current research and scientific studies clearly indicate that CO2 should be treated as a basic or auxiliary raw material that already allows or in the near future will enable the production of a number of goods for further use [1]. Current directions of using carbon dioxide are presented in the figure below Figure 1; [2].
Figure 1: Capture and utilization of CO2 (CCU) pathways (RWGS - reverse water gas shift; F-T - Fischer-Tropsch; DME - dimethylether) [2].
General Overview
Figure 2: Conventional thermal decomposition production of lime (top left) versus STEP direct solar conversion of calcium carbonate to calcium oxide (top right) eliminating CO2 [3].
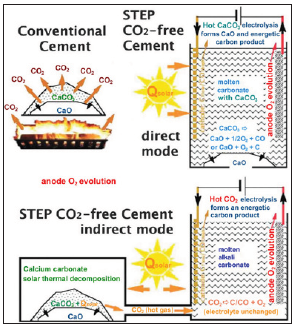
Apart from its negative effects on the climate, CO2 is also a valuable resource, containing carbon - one of the most used and processed elements on Earth. Coal is predicted to continue to dominate power generation for the next 20 years, and since power generation from coal is a significant source of carbon dioxide emissions, the reduction of these emissions is a critical research need. For example, the US Department of Energy’s (DOE) Carbon Sequestration Program, along with related research and development programs throughout the world, continues to make progress toward the goals of lowering the cost of CO2 capture and ensuring that the CO2 can be safely and permanently sequestered in geologic formations in a process know as carbon capture and storage (CCS). Another potential approach to reduce CO2 emissions is CO2 utilization, sometimes referred to as CO2 reuse or carbon capture and reuse (CCR). In the literature there can be find several proposals connected with the perspective use of carbon dioxide as a raw material or elaboration of a new technologies without CO2 emission. Some years ago, there was publish a short communication related to the area of a new molten salt chemistry which allows solar thermal energy to drive calcium oxide production without any carbon dioxide emission. This new approach is called STEP (Solar Thermal Electrochemical Production). In STEP cement limestone undergoes low energy electrolysis to produce lime, oxygen and reduced carbonate without carbon dioxide emission Figure 2; [3].
Reducing our greenhouse gas emissions, while improving the global standard of living, is one of the key fundamental challenges of present century. One of the options that has been proposed to reduce greenhouse gas emissions is the conversion of CO2 and water into fuels and chemicals. This conversion is challenging because the feedstocks have no energy content, carbon dioxide is usually present as a very dilute molecule (about 400ppm in the air) or present as a mixture of gases, and this process requires substantial amounts of energy that must come from another source. Ideally, the energy for this reaction would come from the sun. A somewhat futuristic solution, however with real possibilities of implementation in the near future, is presented in the figure below Figure 3; [4].
Figure 3: Schematic for solar fuels production. Solar fuel feedstocks (CO2, H2O, and solar energy) are captured on-site and/or transported to the solar refinery. Solar energy provides solar utilities in the form of heating, electricity, and photons which are used in the solar refinery to convert CO2 and H2O into fuels. CO2 and H2O are converted to fuels through two principal routes: (1) direct solardriven CO2 reduction by H2O to fuels or (2) solar activation of CO2/H2O to CO/H2, respectively, and subsequent catalytic conversion to fuels via traditional processing (i.e., methanol synthesis or Fischer-Tropsch). The approximate temperature requirements for the solar-driven conversion processes are color-coded (red = high temperature, yellow = ambient temperature) [4].

It should be noted that technologies for the recovery and reuse of carbon dioxide have been known since the second half of the nineteenth century and include three main processes developed in 1869-1922, namely: the synthesis of salicylic acid from sodium or potassium salt of phenol and CO2 (1869), the Solvay process for the synthesis of NaHCO3-Na2CO3 (1882) and the conversion of NH3 and CO2 to urea (1922). With the high-tonnage of urea production, the first two of these technologies are much less exposed as the direction of carbon dioxide management.
Currently, the steam reforming of methane is commonly used in the industrial practice. However, other types of reforming also find practical applications. The sulfur-passivated reforming developed by Haldor Topsøe operates at high carbon concentrations and favorable thermodynamic coke-formation conditions. The process benefits from controlling the amount of hydrogen sulfide in the feed stream that block the carbon nucleation sites on the catalyst through chemisorption of H2S on the step sites of metallic Ni particles. The SPARG (Sulfur PAssivated ReforminG) process is the first technology used, using CH4/CO2 reforming Figure 4; [5,6]. The idea of its development by Sterling Chemical Inc., was the reduction of H2/CO ratio from about 2.7 to 1.8.
Figure 4: Simplified SPARG process flow diagram [5,6].
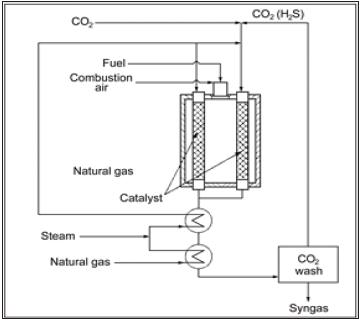
Figure 5: Schematic of the CALCOR standard process [7].

While the SPARG process combines both types of reforming: steam and dry, and its primary goal is to obtain synthesis gas with the appropriate H2/CO ratio, the CALCOR technology (introduced by Caloric GmbH) is implemented mainly in the aspect of obtaining very high CO purity from natural gas or LPG (liquefied petroleum gas). Dry reforming is the basic reaction on which the mentioned CALCOR (CALoric CO Removal) process is based Figure 5; [7].
Conclusion
An important challenge in the near future will be the further development of methods related to the direct use and conversion of CO2 towards useful products, as well as the issue of possible storage of carbon dioxide.
Acknowledgement
This work was supported by the Ministry of Education and Science, Republic of Bulgaria, program Eplus, grant D01-214/2019.
References
- TG (2004) Ultrafast hardening waterproof Portland cement, Russia.
© 2021 Ryczkowski J. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






