- Submissions

Full Text
Progress in Petrochemical Science
On the Formation of Organosulfur Pseudo- Components in the Raw Materials of the Diesel Fuel Hydrotreating Process
Samoilov NA1* and Zhilina VA1
1Department of Petrochemistry and Chemical Technology, Russia
*Corresponding author: Samoilov NA,Department of Petrochemistry and Chemical Technology, Russia
Submission: October 18, 2020;Published: December 01, 2020

ISSN 2637-8035Volume3 Issue5
Opinion
Mathematical modeling of the diesel fuel Hydrotreating process is impossible without a database on the composition and physical and chemical properties of organosulfur components in the feedstock, since the degree of activity of sulfur compounds in hydrogenolysis reactions is different and decreases in the series: mercaptans > sulfides > thiophenes > benzothiophenes > dibenzothiophenes. There are alternative solutions to the problem of describing the composition of Hydrotreating raw materials.
The first option is to identify the most complete set of organosulfur components of diesel fuel and develop a data Bank of possible reaction routes; this solution, in principle, allows the most adequate characterization of Hydrotreating raw materials, but it is the most time-consuming and not always solved from the point of view of the sensitivity of analytical devices. We can note a generalization of the thermodynamic parameters of hydrogenation reactions (entropy and Gibbs energy) for 38 organosulfur components [1]. However, it is quite problematic to implement kinetic experiments to obtain the physical and chemical characteristics of reactions necessary for modeling the process, first of all, the constants of the Arrhenius equation (activation energy and pre-exponential multiplier) due to the microconcentrations of many components in the reaction mixture.
The second option is to formally combine the components of one group of organosulfur substances into a conditional pseudo-component [2-4], but the calculated constants of both the Arrhenius equation and the reaction rate constants themselves are effective and do not allow forming an objective analysis of the reaction process. For example, in [2], the study of diesel fuel desulfurization was performed using the following conditional pseudocomponents: sulfides, ethylbenzothiophenes, propylbenzothiophenes, butylbenzothiophenes, dibenzothiophenes, methyldibenzothiophenes and ethyldibenzothiophenes, but in [3] a different grouping of pseudo-components was used. In [3] also provides an example of representing the composition of organosulfur components in diesel in the form of 2, 3 and 4 pseudo-components, in the latter case they were divided into very easily hydrogenated, easily hydrogenated, difficult hydrogenate and very difficult to hydrogenate without identifying the hydrogenated components.
In this regard, we propose to conditionally divide the initial diesel fuel into N narrow fractions, in each of which the set of organosulfur components is considered as a pseudocomponent, for which the rate constant of the hydrogenation reaction can be easily determined experimentally. With this representation of pseudo-components, the results of mathematical modeling will be determined only by the number of narrow fractions. We have performed mathematical modeling of diesel fuel hydrotreatment taking into account from two to 16 pseudo-components. The model of Hydrotreating process kinetics in this case has the form of a system of equations
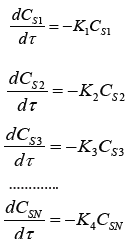
where CSI and Ki, respectively, are the concentration of the organosulfur pseudo-component and the rate constant of the i-th reaction.
References
- Afanasyeva YI, Krivtsova NI, Ivanchina ED, Zanin IK, Tataurshchikov AA (2012) Development of a kinetic model of the diesel fuel hydrotreating process. Izvestiya Tomsk Polytechnic University 321(3): 121-125.
- Krivtsova NI, Krivtsov EB, Ivanchina ED, Golovko AK (2013) Kinetic regularities of hydrodesulfurization of diesel fraction. Fundamental Research 8(3): 640-644.
- Tang X, Li S, Yue C, He J, Hou J (2013) Lumping kinetics of hydrodesulfurization and hydrodenitrogenation of the middle distillate from Chinese shale oil. Oil Shale 30(4): 517-535.
- Bannatham P, Teeraboonchaikul S, Patirupanon T, Arkardvipart W, Limtrakul S, et al. (2016) Kinetic evaluation for hydrodesulfurization via lumped model in a trickle-bed reactor. Industrial & Engineering Chemistry Research 55(17): 4878-4886.
© 2020 Samoilov NA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






