- Submissions

Full Text
Progress in Petrochemical Science
Carbon Dioxide Recycling for Fuels and Chemical Products
V Beschkov*, E Razkazova Velkova and L Liutzkanov
Institute of Chemical Engineering, Bulgarian Academy of Sciences, Bulgaria
*Corresponding author: V Beschkov, Institute of Chemical Engineering, Bulgarian Academy of Sciences, Bulgaria
Submission: October 10, 2020;Published: November 17, 2020

ISSN 2637-8035Volume3 Issue5
Abstract
The problem of the adverse effect of greenhouse gases released in the atmosphere became a global one in the recent years due to the caused probable climate changes. The mostly spread greenhouse gases are methane and carbon dioxide emitted by agriculture (methane), transport, industry and households (carbon dioxide). Carbon dioxide is considered as a big threat for climate changes because of its very powerful emissions all over the world. There are different ways for remedy of this global threat. First, it is to increase the energy efficiency to spend less carbon containing fuels in transport and industry. Another way is to replace, at least partially, the carbon containing fossil fuels by renewable ones, like wind, solar energy and waterpower, or by recyclable biomass. The third one is to recycle the emitted carbon dioxide to fuels (e.g. methane, synthesis gas, light hydrocarbons) and/or useful chemical products (methanol, formic acid, etc.). Mostly the carbon dioxide recycling is based on endothermic processes requiring input of energy thus polluting atmosphere with carbon dioxide in the general case. That is why a carbon-free sources of energy must be applied. Fuel cell applications seem promising to such a purpose.This minireview presents a comparison of the available data for reverse carbon dioxide conversion to methane and organic compounds.
Keywords: Carbon dioxide recycling;Fuels;Chemical production;Fuel cell application
Introduction
Methane has various practical applications as fuel, raw material for energy production in fuel cells, for nitrogen-containing fertilizers, syngas production for synthetic fuel applications by the Fischer-Tropsch process, etc. However, in all of these cases the resulting waste is carbon dioxide, assumed as harmful pollutant of atmosphere, leading to greenhouse effect and climate changes.
There are different ways to remediate the adverse effects of carbon dioxide on atmosphere: emission minimization, use of renewable energy sources and carbon dioxide recycling. Emission minimization is rendered to two main approaches: by improvement of energy efficiency in different ways or by replacement of fossil fuels by renewable ones, i.e. solar or wind energy, biomass.
The use of renewables is to replace, at least partially, the fossil fuels (oil, coal and natural gas) by renewable energy sources, like solar and wind energy and biomass as well. The latter enables to relate the present carbon dioxide emissions with the carbon cycle, closed by the existing vegetation by photosynthesis. Such fuels produced from biomass are biogas (a mixture of methane and carbon dioxide) generated by anaerobic digestion of organic waste, ethanol, produced by fermentation of carbohydrates, and biodiesel, produced by transesterification of lipids. Within this approach the biogas applications as a fuel and source for syngas production by dry reforming are considered.
All approaches of biomass utilization as renewable energy resource end with the inevitable carbon dioxide release due to combustion. On the other hand, the vegetation growth requires energy expenses also associated with carbon dioxide release. That is why, recycling of abiotic carbon dioxide is the main goal of the present scientific research and in the near future. This mini review presents a comparison of the available data for reverse carbon dioxide conversion to methane and organic compounds, including own data.
Literature Review
An attractive approach is to convert the waste carbon dioxide into organic chemicals by catalytic processes. Such products are methanol, formic acid and methane. The problem
is the high thermodynamic stability of carbon dioxide and the endothermic processes of carbon dioxide reduction to organic chemicals. There are different ways to overcome this drawback: to use solar energy, other renewables like wind and water energies and fuel cell applications.
There are different methods for carbon dioxide recycling by chemical catalysis [1,2]. Some of them results in production of urea, methane (by Sabatier & Senderens reaction), synthesis gas by dry reforming [3] with further applications, light fuels by Fischer- Tropsch process and other catalysts [4], dimethylether for fuel additive, acrylic acid, iso-cyanates, etc.:
2N3+CO2 = CO(NH2)2+H2O (urea) CО<2+2H2 = CH4+H2O (Sabatier’s reaction) CH4+CO2 = 2CO+2H2 (dry reforming) (1) 2CO+4H2 = 2CH3OH↔CH3OCH3 H2C=CH2+CO2 = H2C=CH-COOH; RNH2+CO2 = RNCO+H2O
Unfortunately, all these reactions require high temperature and pressure and therefore high input of energy and release of carbon dioxide. That is why other sources of energy avoiding carbon dioxide release are necessary. The first approach is the photocatalytic reduction of gaseous carbon dioxide using solar energy [5-9]. Different products are obtained - carbon monoxide [5], methanol [6,7], light hydrocarbons [8] or methane [9]. The main problem in all these cases is the low yield of the products.
Another way is the electrochemical reduction of carbonate in aqueous media. The first step is to capture carbon dioxide by alkaline agents as carbonate and to use it further to produce chemicals or energy. The following cathode reactions are involved:
CO2+2H++2e- = CO+H2O CO2+2H++2e- = HCOOH CO2+4H++4e- = HCHO (2) CO2+6H++6e- = CH3OH+H2O CO2+8H++8e- = CH4+2H2O
There are communications in the literature for methanol production by electrochemical reduction of carbon dioxide [10] or for other liquid fuels [11]. There are some efforts for combined electrochemical and catalytic reduction [12-14].
Fuel Cell Application –Own Data
A sketch of the fuel cell operation for the case of carbon dioxide (or bicarbonate and carbonate respectively) reduction is shown in Figure 1. The expected cathodic reactions are described above, Eqs (2).
Figure 1: A principal sketch of a fuel cell operation with aqueous carbonate as oxidizer.
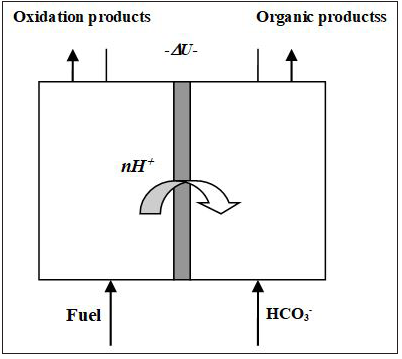
Figure 2: Flowsheet of fuel cell application for CO2 recycling.
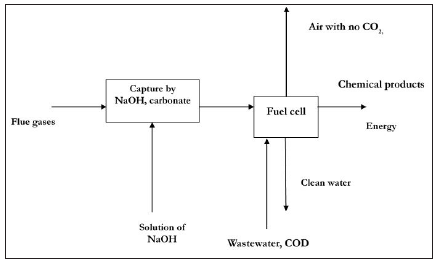
Various reductors can be used as a fuel. For example, wastewater with high COD content could be applied. Hence, this process will have multiple effect: carbon dioxide removal and recycling, energy generation for self-consumption and wastewater treatment. The principal technology flowsheet is shown in Figure 2. The fuel cell consisted of two square parallel plates made out of sintered graphite with area of 100 sq.cm each. A Fumitem anion exchange membrane was used as separator. The slot of the anode and cathode compartments was 5 mm each. The cathodic space was packed with carbon grains containing catalyst prepared by us: manganese oxide (catalyst 1) and tin (catalyst 2). The experiments were carried out in aqueous media containing sodium bicarbonate at initial concentrations from 0.02 to 0.4M. As an electron donor (fuel) solution of sodium sulfide with 240 mg dm-3 was used. Both batch and continuous processes for the cathodic space were tested.
The experiments consisted in parallel measurements of the cell potential and the current. The power density was calculated from the set current density and the generated electro-motive force. The electrochemical reduction of bicarbonate was measured according to the Faraday law and compared to the data of titrimetrical analysis. The NMR-analyses showed qualitatively that the single product of carbon dioxide reduction was formic acid (or formiate), cf. Eqs (3). The concentrations of formic acid were determined by HPLC.
HCO3-+2H++2e- = HCOO-+H2O
CO32-+3H++2e- = HCOO-+H2O (3)
Better quantitative results were obtained with catalyst 2.
Discussion
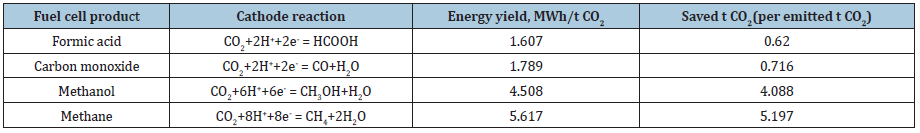
The discussion is dedicated to the energy balance for carbon dioxide recycling by the proposed method: capturing by sodium hydroxide electrochemical reduction with electricity production in a fuel cell and wastewater treatment. The energy and the carbon dioxide release for production of NaOH are included into the energy balance. The enthalpies for the organic chemical products necessary for the calculations are the theoretical ones. The comparison of the energy yields and CO2 saving when different end products are obtained is shown Table 1. Hence, there is a multiple effect of the fuel cell approach: first, carbon dioxide removal; next, value-added chemical products are produced by CO2 recycling; wastewater treatment; and last, but not least, electricity is produced. Of course, this electric energy is not of the crucial importance, but it can be used for feeding the fuel cell equipment.
The level of the fuel cell utilization depends on the cathode reactions listed in Table 1 and Eqs (2). The more profitable reactions of methanol and/or methane formation depend on the catalyst selected. There is the goal of our further efforts.
Table 1: Energy yields and saved carbon dioxide at fuel cell application.
Acknowledgement
This work was supported by the Ministry of Education and Science, Republic of Bulgaria, program Eplus, grant D01-214/2019.
References
- Quadrelli EA, Centi G, Duplan JL, Perathoner S (2011) Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 4(9): 1194-1215.
- Sankaranarayanan S, Srinivasan K (2012) Carbon dioxide- A potential raw material for production of fuel, fuel additives and bio-derived chemicals. Indian Journal of Chemistry 51A(9-10): 1252-1262.
- Damyanova S, Pawelec B, Arishtirova K, Fierro JLG (2011) Biogas reforming over bimetallic PdNi catalysts supported on phosphorus-Modified alumina. International Journal of Hydrogen Energy 36(17): 10635-10647.
- Wang J, Zhang A, Jiang X, Songa C, Guo JX (2018) Highly selective conversion of CO2 to lower hydrocarbons (C2-C4) over bifunctional catalysts composed of In2O3-ZrO2 and zeolite. Journal of CO2 Utilization 27: 81-88.
- Junfu L, Baozhu C (1992) Photoelectrochemical reduction of carbon dioxide on a p+/p-Si photocathode in aqueous electrolyte. J Electroanal Chem 324: 191-200.
- Kumar B, Smieja JM, Kubiak CP (2010) Photoreduction of CO2 on p-type silicon using Re(bipy-But )(CO)3Cl: photovoltages exceeding 600 mV for the selective reduction of CO2 to CO. J Phys Chem C 114: 14220-14223.
- Gondal MA, Lais A, Dastageer MA, Yang D, Shen K, et al. (2017) Photocatalytic conversion of CO2 into methanol using graphitic carbon nitride under solar, UV lase and broadband radiations. International Journal of Energy Research 41(14): 2162-2172.
- Tahir M, Amin NAS (2013) Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Conversion and Management 76: 194-214.
- Sastre F, Puga AV, Liu L, Corma A, García H (2014) Complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation. J Am Chem Soc 136(19): 6798-6801.
- Zhang W, Qin Q, Dai L, Qin R, Zhao X, et al. (2018) Electrochemical reduction of carbon dioxide in methanol on hierarchical Pd/SnO2 nanosheets with abundant Pd-O-Sn interfaces. Angewandte Chemie 57(30): 9475-9479.
- Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (2009) Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem Soc Rev 38: 89-99.
- Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, et al. (2014) Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J Am Chem Soc 136(40): 14107-14113.
- Zhu Y, Zhang S, Ye Y, Zhang X, Wang L, et al. (2012) Catalytic conversion of carbon dioxide to methane on ruthenium-cobalt bimetallic nanocatalysts and correlation between surface chemistry of catalysts under reaction conditions and catalytic performances. ACS Catalysis 2(11): 2403-2408.
- Qiao J, Liu Y, Hong F, Zhang J (2014) A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43(2): 631-675.
- Hara M, Onaka Y, Kobayashi H, Fu Q, Kawaguchi H, et al. (2013) Mechanism of electromethanogenic reduction of CO2 by a thermophilic methanogen. Energy Procedia 37: 7021-7028.
- Min S, Yong J, Yao Z, Ping G, Daping L (2013) Coupled bioelectrochemical system for reducing CO2 to simple organic compounds in the presence of H2. Chinese J Appl Environ Biol 19(5): 827-832.
- Li H, Opgenorth PH, Wernick DG, Rogers S, Wu TY, et al. (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335(6076): 1596.
- Wee JH (2010) Contribution of fuel cell systems to CO2 emission reduction in their application fields. Renewable and Sustainable Energy Reviews 14(2): 735-744.
© 2020 V Beschkov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






