- Submissions

Full Text
Progress in Petrochemical Science
Advances in Syngas Production Technologies: Catalysis and Engineering
Makarand R Gogate*
Independent Consultant for Ch.E Education and Research, India
*Corresponding author: Makarand R Gogate, Independent Consultant for Ch.E Education and Research, India
Submission: June 05, 2020;Published: August 20, 2020

ISSN 2637-8035Volume3 Issue4
Opinion
Methane is the principal constituent of natural gas and constitutes over 90% (v/v) by volume, regardless of the source. Coal, oil, and natural gas have traditionally been the 3 fossil fuels of choice for further conversion and upgrading to fuels and fuel additives, chemicals, and petrochemicals, and for generation of electric power. The advent of “Fracking”, a technology first commercialized in the United States around 2008, made it possible to harvest and recover huge quantities of shale gas and associated gas liquids, trapped within tight pore spaces of shale rock deposits or coal bed methane, considered to be 2 unconventional sources of methane [1-3], other than gas hydrates. The production of domestic natural gas saw a hugh spike in about 2008 (about 1Tnm3) and is expected to grow by up to 44% by 2035. The U.S. is now the world’s largest producer of natural gas, and the cost of natural gas is at about the lowest it has been in over 2 decades, at $1.85/MM BTU. The historical trends in the production and price of domestic natural gas, 1900 onwards to 2020, is shown in Figure 1. Instructively, the hugh spike in the production capacity of natural gas is clearly seen around 2005-2010, which coincides with the advent of new “Fracking” capacity in the United States [4].
Figure 1: Historical trends in price of domestic natural gas, 1900 onwards to 2020 [4].

Not surprisingly, natural gas surpassed coal (in 2007) for the largest installed electricity generation capacity in the United States. In addition, the E.I.A. estimates that the unconventional resource base (primarily shale and coal bed methane) of natural gas is around 65Tnm3, out of which about 5Tnm3 are considered to be “proven” reserves, i.e., recoverable under the current economic and environmental conditions [4]. Natural gas, a versatile fuel feedstock, with a high calorific value - one with the lowest C footprint on account of its highest H:C ratio – is primarily used for electricity generation, and for home heating/cooking applications. More than 90% of U.S. domestic production is burned to create energy, for heating, cooking, and transportation purposes, or for generation of electric power (for residential and commercial use). The use of natural gas as a chemical feedstock for further conversion into fuels/fuel additives, chemicals, and petrochemicals, is still very limited. The reason for this is primarily economic in nature. Most natural gas wellhead locations/deposits are found in remote, inaccessible locations. Natural gas, a vapor under ambient conditions, has a very low mass and volumetric energy density, and is difficult and uneconomical to transport over long distances, using gas pipelines, or even LNG tanker trailers. Unfortunately, thus, apart from conversion to synthesis gas, hydrogen cyanide, acetylene, and chlorinated hydrocarbons, methane conversion pathways are not yet cost-competitive to oil-based fuels and chemicals/petrochemicals. However, natural-gas based indirect liquefaction technologies, based on syngas, offer a critical potential avenue (to reduce our dependence on oil and to reduce the C footprint), for further high-volume growth and market share in syngas-based bulk chemicals. The top 3 chemical products, based on natural gas-based syngas, are, ammonia (worldwide capacity 175MMtpa, 11MMtpa U.S.), methanol (110MMtpa, 4.5MMtpa U.S.), and F.-T.-based synfuels products (over 220,000bpd). While the U.S. capacity of the top-3 chemicals above is still a very small fraction of the worldwide capacity, more than a dozen methanol mega projects are currently in various stages of planning, design, and construction, all along the U.S. gulf coast. Natural-gas based syngas is an ideal feedstock for production of above 3 chemicals, as it affords a stoichiometric inlet H2:CO ratio of 2-2.5, directly possible with both conventional steam reforming and autothermal reforming (ATR), without need for any additional shift conversion [5-8].
In this article, we offer an insightful analysis of the current status of syngas production technologies and assess future projections and forecast for the industry. Steam reforming of natural gas is a conventional and now mature technology, for synthesis of “syngas”, a mixture of CO, CO2, and H2 [9-12]. In steam reforming, CH4 reacts with steam to reform CH4 into a mixture of CO, CO2, and H2, as given below:
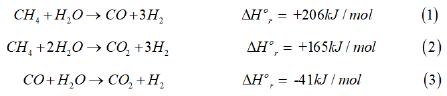
The synthesis reactions are highly endothermic and thus limited by chemical equilibrium; heat is supplied to the reformer tubes (in a vertical, parallel arrangement), by combustion of natural gas inside a firebox. The product gases leave the reformer unit at 855 oC and 2MPa. In industrial practice, heat is recovered from this gas stream by a series of heat exchange operations.
As noted above, only 2 of the 3 reactions above are independent; the H2:CO ratio for the overall product gas is between 2-2.5. The kinetic studies on a commercial Ni/γ-Al2O3 or a ceramic support indicate that it is the reforming of CH4 to CO and the water gas shift reaction that take place under industrial conditions. The dry reforming reaction can also be postulated to occur in the overall series of reactions, as follows [13]:
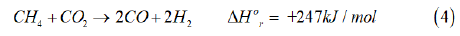
As discussed above, steam reforming of natural gas is a mature process technology, and discussed extensively in several recent reviews [6,7,9-12].
Steam reforming is catalyzed by Group VIII transition metals, including Ru, Rh, Ir, and Ni. While extensive experimental and theoretical studies (DFT calculations, scaling relationships, and microkinetic models) show that Ru and Rh are the most active transition group metals for steam reforming, Ni-based catalysts are almost exclusively used in the chemical industry due to high price of Ru and Rh metals. Ir is also an excellent choice as a catalyst, with identical activity and selectivity profiles.
In industry, Ni catalysts are highly susceptible to severe deactivation, by,
- sintering and particle growth,
- selective poisoning by trace impurities in feed gas, including As, Pb, S, and P, and
- C deposition and pore blockage to active sites, by Boudouard reaction, and methane cracking/decomposition, at the high temperatures (>823-1073 K) encountered in steam reforming [14].
Of the three causes mentioned above, sintering and particle growth occur by atom-by-atom translation and migration from one particle to other, a mechanism termed as Ostwald ripening. It has been proposed that Ni surface atoms are quite labile at the high process temperatures and migrate over surfaces by formation of Ni-H and Ni-OH entities. However, promoters like Au and K that selectively bind to active sites (both terrace and step sites) and form surface alloys are shown to impart both sintering and coking resistance and extend catalyst lifetimes in industrial practice. For further reading on causes and consequences of catalyst deactivation, the reader is referred to some recent reviews on this topic [9,12,15-17].
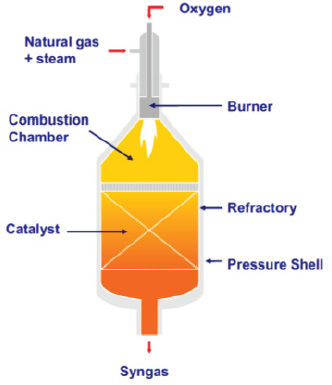
Compared to conventional steam reforming, autothermal reforming (ATR) is a transformational advance in syngas technology, and consists of a combination of a combustion chamber at the reactor inlet in series with a packed bed further away from the inlet, to improve the overall reactor efficiency and operational flexibility [5,12,18]. Natural gas and steam, fed to the combustion zone in presence of O2, at temperatures of ~1300 oC (and higher in the flame core, right at burner entrance mounted ahead of the combustion chamber), undergo homogeneous gas phase reactions, including steam reforming and water gas shift (among other radical reactions). The final conversion takes place in the catalyst bed, and the product gases leave the reformer, at chemical equilibrium, between 850-1100 oC. The ATR operation is very flexible, uses low steam/CH4 and O2/CH4 ratios (sub-stoichiometric), and produces syngas with a wide range of H2:CO ratios, of both CO-rich and H2-rich type. In addition, the ATR operation is typically soot-free and particulate-free. The operational concept of the ATR system is illustrated in Figure 2. Like conventional reforming, Ni-based catalysts are used, but with refractory or spinel support which have high thermal and mechanical strength/stability, to withstand the very high temperatures of operation [9]. Both α-Al2O3 and MgAl2O4 spinel oxide are industrially used as supports.
Figure 2: Illustration of the ATR reactor operation [9].
In catalytic partial oxidation (CPO), still an exploratory technology in nature, the hydrocarbon feed and oxidant (O2) are mixed in the inlet zone of the CPO reactor, and passed over the packed bed of catalyst. Distinct with the ATR operation, the CPO reactor design does not incorporate a burner ahead the catalyst zone. The steam reforming and water gas shift reactions take place over a noble metal-based catalyst, and the product gases typically leave the reactor exit at chemical equilibrium, at temperatures typically >1100 oC. Noteworthy research advances in this area include the development of ms-time scale contact reactors, which incorporate design allowances that circumvent needs for pre-heating, and steam addition [19,20]. Regardless, CPO technology is unlikely to have commercial merit, mainly due to safety concerns, associated with handling of the hydrocarbon-O2 flammable mixture at the inlet. The feed inlet is also designed to be outside the auto-ignition temperature of the mixture, which inherently leads to a higher consumption of O2.
References
- Bruijnincx PC, Weckhuysen BM (2013) Shale gas revolution: an opportunity for bio-based chemicals. Angewandte Chemie International Edition 52(46): 11980-11987.
- Wang Q, Chen X, Jha AN, Rogers H (2014) Natural gas from shale formation - the evolution, evidences, and challenges of shale gas revolution in the United States. Renew Sustain Energy Rev 30(C): 1-28.
- Makogon YF, Holditch SA, Makogon TY (2007) Natural gas-hydrates - a potential energy source for the 21st J Petro Sci Eng 56(1-3): 14-31.
- Energy Information Administration (2012).
- Rostrup Nielsen JR, Christiansen LJ (2011) Concepts in syngas manufacture, Imperial College Press, London, UK.
- Lu K, Song C, Subramani V (2010) Syngas and hydrogen production and purification technologies, Wiley, Hoboken, New Jersey, USA.
- Rostrup Nielsen JR (2008) Handbook of heterogeneous catalysis. In: Ertl G, Knözinger H, Schüth F, Weitkamp J (Eds.), Wiley-VCH, Weinheim, Germany.
- Tillerson R (2005) Leading the way fundamentals of gas-to-liquids. In: 2nd (edn), Petroleum Economist, London, UK.
- Petersen AK, Dybkjaer I, Ovesen CV, Schjodt NC, Sehested J, et al. (2011) Natural gas to syngas: Catalysts and catalytic processes. J Nat Gas Sci Eng 3(2): 423-459.
- International energy agency (2012) Golden rules for a golden age of gas.
- Wood DA, Nwaoha, C, Towler BF (2012) Gas-to-liquids: a review of an industry offering several routes for monetising natural gas. J Nat Gas Sci Eng 9: 196-208.
- Rostrup JR (1993) Production of synthesis gas. Catal Today 18(4): 305-324.
- Bradford MJ, Vannice MA (1993) CO2 reforming of CH4. Catal Rev Sci Eng 41(1): 1-42.
- Horn R, Schlögl R (2015) Methane activation by heterogeneous catalysis. Catal Lett 145: 23-39.
- Ratnasamy C, Wagner JP (2009) Water-gas shift catalysis. Catal Sci Rev Eng 51(3): 325-340.
- Rasmussen FB, Sehested J, Teumssen HT, Molenbroek AM, Clausen BS (2004) Sintering of Ni/Al2O3 catalysts studied by anomalous small angle X-ray scattering. Appl Catal A 267(1-2): 165-173.
- Sehested J, Carlsson A, Janssens TW, Hansen PL, Datye A (2001) Sintering of Nickel steam reforming catalysts on MgAl2O4 J Catal 197(1): 200-209.
- Reyes SC, Sinfelt JH, Feeley JS (2003) Evolution of processes for syngas production: Recent developments in an old technology. Ind Eng Chem Res 42(8): 1588-1597.
- Schmidt LD (2000) Millisecond chemical reactions and reactors. Stud Surf Sci Catal 130: 61-81.
- Specchia S, Vella L, Montini T, Fornasiero P (2011) Syngas production by short contact-time catalytic partial oxidation of methane, Hydrogen production: Prospectives and processes. In: Honnery DR, Moriarty D (Eds.), (1st edn), Nova Science Publishers Inc., Hauppauge, New York, USA, pp. 95-139.
© 2020 Makarand R Gogate. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






