- Submissions

Full Text
Progress in Petrochemical Science
Hydraulic Fracturing (“Fracking”) and Rebirth of United States Chemical Industry
Makarand R Gogate*
Independent Consultant for Ch.E Education and Research, India
*Corresponding author: Makarand R Gogate, Independent Consultant for Ch.E Education and Research, India
Submission: June 05, 2020;Published: June 22, 2020

ISSN 2637-8035Volume3 Issue3
Opinion
The use of hydraulic fracturing the extract oil and gas from the earth, dates back to 1940s, but only in the last decade or so, has the word “Fracking” become a buzzword. Fracking alludes primarily to the shale gas boom in the United States, since about 2008, and also refers to the process - the high pressure injection of water, chemicals, and sand into the shale rock deposits – to release gas and oil trapped within shale rock by fracturing it, to harvest stores of gas and oil, previously unfeasible to access or recover.
As stated above, Fracking became commercial in the United States in 2008, first at the Bakken Shale deposits in North Dakota, and later at Barnett Shale Basin in Texas. The production of domestic natural gas thus saw a high spike in about 2008, and is expected to grow by up to 44% by 2035. For example, the domestic production was at 17Tn cu ft/year in 2008, and now stands at 27Tn cu ft/year (2017), an increase of 59%. As a result, the price of domestic natural gas (Henry Hub Index) was $15.46/MM BTU (2008 basis), and has steadily declined to the current $1.85/MM BTU (2020). The historical trends in the natural gas prices are illustrated in Figure 1; [1]. Not surprisingly, the oil prices, seen historically to trend directly with price of natural gas, have also steadily declined: Oil prices peaked to over $110/bbl (2008) - when the cost of gasoline was over $4/gal -- and have also steadily declined to $30/bbl, as of today. Fracking is a uniquely and hugely successful American story, that, by safely unlocking America’s abundant natural resources, has created millions of jobs, reduced energy prices (oil and natural gas), and also brought cleaner air by significantly reducing greenhouse gas emissions (to lowest levels in 25 years), and transformed the United States back into a global superpower [2].
The global chemical industry is heavily dependent on stable and inexpensive sources of fossil fuel feedstock, such as crude oil and natural gas. However, increasing utilization and exploitation of crude oil in the 1980s and 1990s, fueled by cheap prices and plentiful supply, led to eventual price instability, and supply chain issues. Consequently, the United States chemical industry was in decline in the late 1990s and early 2000s. Many chemical producers were thus faced with either using syngas as a chemical feedstock - a mixture of CO, CO2, and H2, from domestic coal, biomass, and natural gas, or disassemble existing chemical plants and facilities altogether and relocate them outside of the United States.
As discussed above, a major technological breakthrough, called as “Hydraulic Fracturing”, or, “Fracking”, for short, occurred in about 2008, which made possible the very economical recovery of natural gas and gas liquids associated with high amounts of shale oil deposits in the United States. The shale gas boom, as it is commonly called now, led to gigantic increases in the production/recovery of both natural gas and gas liquids in the United States. This has clearly led to a “rebirth” of the U.S. chemical industry, and the business of industrial chemistry is growing, with a very rosy outlook to the future.
It is a very opportune time now for the U.S chemical industry, and clearly behooves on us to develop cost-effective process alternatives, due to the strong interest and economic incentives of shale gas. Currently, the catalytic upgrading of methane to value-added chemicals proceeds via the indirect liquefaction route. The resulting syngas is then converted into a host of chemicals and chemical feedstocks, including methanol, ethanol, higher alcohols, light olefins, acrylonitrile, ethylene oxide, propylene oxide, 1,3 butadiene, etc. However, the steam reforming of natural gas is a strongly endothermic and an energy-intensive process, and requires a large energy input. The traditional thrust has therefore been to find process alternatives to steam reforming, or to use syngas from coal gasification as an alternative. Two approaches for the indirect conversion of methane into liquid hydrocarbons are now practiced on an industrial scale: methanol-to-gasoline (MTG) process and Fischer-Tropsch (FT) synthesis [3-8]. It is imperative at this time to enable transformational thinking and pursue transformational discoveries in methane functionalization, towards direct conversion of methane to value-added chemicals. Figure 2 shows the various approaches and pathways for the direct conversion of methane and ethane to value-added chemicals.
As stated above, the plentiful availability of the low-cost natural gas feedstock allows a transformational opportunity to lower the C footprint of the chemical industry. Methane (CH4) has the highest H:C ratio of any fuel, and the highest calorific value, but from a chemical standpoint, the linear single C-H bonds in a perfectly tetrahedral symmetry in methane have a very high bond strength (over 440kJ/mol) and are very difficult to break. Because it a saturated linear hydrocarbon, it is very difficult to activate the C-H bonds, except in very harsh environments, such as very high temperatures (>1100 K), highly oxidative conditions, or highly acidic/alkaline ones. For these reasons, the direct and selective conversion of methane to higher-value chemicals is often considered to be the “Holy Grail” of experimental/theoretical research in heterogeneous catalysis and catalytic chemistry. Despite these difficulties and the chemical recalcitrance of methane, several pioneering technologies for direct catalytic upgrading of methane have been developed. This bodes very well for the United States economy for the foreseeable future. We consider 2 such promising technologies in the following discussion.
The direct, selective oxidation of methane to methanol is often considered to be a “dream reaction” or a “dream process”. The direct oxidation of methane to methanol (and formaldehyde) can be represented as:
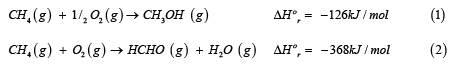
It is interesting to note that both oxidative conversion processes are thermodynamically feasible (-ve standard heats of reaction and Gibbs free energy of reaction), but suffer from very low per-pass selectivity and yield. Based on theoretical calculations, maximum single-pass yield to methanol is about 8%, and that to formaldehyde is even lower, at 3%. For further details, we point the interested reader to some recent reviews of the direct methane to methanol (DMTM) process [9-11].
While the DMTM process, as discussed above, is considered to be a “dream process” - from a research standpoint -- for direct methane upgrading, the oxidative coupling of methane (OCM) technology is perhaps one of the most promising technology avenues for direct upgrading to higher-value chemicals, but some technical impediments remain, for its successful commercial-scale operation and practice [12-15]. From a technological standpoint, the low selectivity to ethylene makes the product separations process (from methane and co-products) quite cost-prohibitive. Further, ethane is less expensive than methane in today’s market, which makes the technology unattractive. The OCM technology is also very highly exothermic and severe heat and mass transport gradients occur over the particle/pellet scale in packed bed reactors. However, these two technical impediments, i.e., the strong correlation between methane conversion and C2 selectivity, which limits the per-pass yield to <30%, and the operational challenges in reactor design, are not to be viewed as impediments but as opportunities for further research innovation and enhancements. For example, newer reactor designs, based on the principle of reactive distillation or membrane separations, i.e., selective physical removal of products, including ethane and ethylene, from the reactor, to break the per-pass yield barrier of <30%, can lead to technological breakthroughs, which may increase commercial viability and appeal of the OCM process [12,15].
References
- US Energy Information Administration (2020) Website.
- Independent Petroleum Association of America (2020) Website.
- Glasser D, Hildebrandt D, Liu X, Lu X, Masuku CM (2020) Recent advances in understanding the Fischer-Tropsch synthesis. Curr Opin Chem Eng 1: 296-302.
- Dry ME (2002) The Fischer-Tropsch process: 1950-2000. Catal Today 71(3-4): 227-241.
- Anderson RB (1984) The Fischer-Tropsch synthesis. Academic Press, New York, USA.
- Keil FJ (1999) Methanol-to-hydrocarbons: Process technology. Micro Meso Matls 29: 49-66.
- Chang CD (1983) Hydrocarbons from methanol. Catal Rev Sci Eng 25(1): 1-118.
- Chang CD, Silvestri AJ (1979) Process for manufacture of gasoline. US Patent 4,138,442, Assigned to Mobil Oil Corporation, New York, USA.
- Arutyunov V (2014) Direct methane to methanol: Foundations and prospects of the process. Elsevier, Amsterdam, The Netherlands.
- Holmen A (2009) Direct conversion of methane into fuels and chemicals. Catal Today 142: 2-8.
- Gesser HD, Hunter NR, Prakash CN (1985) The direct conversion of methane to methanol by controlled oxidation. Chem Rev 85(4): 235-244.
- Cruellas A, Melchiori T, Galluci F, van sint Annaland M (2017) Advanced reactor concepts for oxidative coupling of methane. Catal Rev Sci Eng 59(3): 234-294.
- Schwach P, Pan X, Bao X (2017) Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem Rev 117(13): 8497-8520.
- Zavyalova U, Holena, M, Schlogl R, Baerns M (2011) Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high-performance catalysts. Chem Cat Chem 3: 1935-1948.
- Sinev M Yu, Fattakhova ZT, Lomonosov VI, Gordienko, Yu A (2009) Kinetics of oxidative coupling of methane: Bridging the gap between comprehension and description. J Nat Gas Chem 18: 273-287.
© 2020 Makarand R Gogate. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






