- Submissions

Full Text
Progress in Petrochemical Science
Hypothetical Research Note: Artificial Dark Photosynthesis Models for Capturing Carbon Dioxide into Fuels
Tomonori Kawano1,2,3*, Diego Comparini1,2, Omi Uchimura4,5 and Kazuya Uezu1
1 Faculty of Environmental Engineering, The University of Kitakyushu, Japan
2 University of Florence LINV Kitakyushu Research Center, Japan
3 Paris Interdisciplinary Energy Research Institute (PIERI), France
4 SARAS Co. Ltd, Japan
5 Royal Cooperation, Japan
*Corresponding author: Tomonori Kawano, Department of Life and Environment Engineering, The University of Kitakyushu, Japan
Submission: April 05, 2018;Published: September 12, 2018

ISSN 2637-8035Volume2 Issue5
Introduction
Our team at International Photosynthesis Industrialization Research Center (IPIRC), University of Kitakyushu, aims to uncover and develop the systems for sustainable yield of energy such as fuels through science and engineering of both natural and artificial photosynthesis under collaborations with industries and foreign institutions. The natural systems under collaborations include projects with European institutions such as Univ. Paris-Diderot (France) and Univ. of Florence (Italy) for bio-oil production from a Mediterranean halophyte grown on the non-agricultural lands and a collaborative project held in Kitakyushu city, for producing the jet fuel using marine micro-algae run by Electric Power Development Co., Ltd, as we have developed an efficient protocols for extraction of bio-oils from bio-materials [1]. On the other hand, artificial systems under collaborations include a project (with Royal Corp. Ltd. and Sankyu Inc.) for developing a photosynthetic carbon fixation (dark reaction)-mimicking mechanism for absorption and fixation of carbon dioxide (CO2) followed by carbon utilization and production of fuels (synthetic diesel).
As the aforementioned Royal Corp. Ltd. has launched, under the auspices of the presidential office of the government of Philippine, a pilot plant in the suburb of Manilla, Philippine, empirically aiming for the process allowing the atmospheric CO2 to be captured in the oil/methanol/water mixture for further direct reaction yielding hydrocarbons under mild conditions in the presence of several types of catalysts; the priority mission for our academic team was to mimic the phenomena in the laboratory bench-top scale based on the working hypothesis possibly explaining the phenomena. Therefore, our team has been intensively engaged in development of novel artificial photosynthetic model reactions which allow reversible uptake and/or release of atmospheric CO2 [2], followed by production and/or consumption of simple organic molecules in laboratory scale in vitro systems. The approach towards such artificial photosynthesis research involves the development and use of organic and inorganic catalysts (artificial enzymes) employed by analogies to redox active natural enzymes or proteins. Here, we briefly present the hypothetical model for enhanced capturing of atmospheric CO2 through finely mixed oil/methanol/water system containing model inorganic catalyst mixture, and the minimal supportive data to date [3].
Working Hypothesis for Carbon Capture and Utilization for Fuel Production based on the Mass-Balance Analysis with the Pilot Plant (Royal Cooperation, Manilla, Philippine)
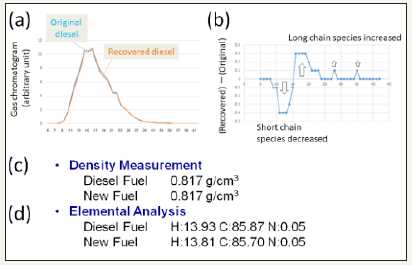
In Philippine, a model pilot plant originally designed for waterbased refinement (removal of air-pollution precursors) of diesel fuel was installed (Figure 1). Interestingly, several engineers at the Royal Cooperation have noticed that the volume of diesel recovered after aquatic washing process proceed the original volume of the fuel loaded onto the system, suspecting that there would be a phenomenon with unknown mechanism are ongoing. Chemical analyses (Figure 2) performed for comparison of the original diesel and recovered diesel suggested that quality of tow samples is almost identical having identical density and similar elemental compositions. However, gas chromatography suggested that hydrocarbons with slightly elongated chains could be observed in the recovered sample possibly suggesting the possible relationship between the enhanced yield of fuel and elongation of the carbon chain in the hydrocarbons. There would be two major reactions during CO2 utilizing production in the pilot plant producing synthetic diesel, namely, [2] initial start-up reaction requiring template hydrocarbons and methanol yielding intermediate hydrocarbons with concomitant elimination of water molecules, and [1] massive reactions lengthening the hydrocarbon chains requiring the template hydrocarbons (and the intermediate hydrocarbons), CO2, and water molecules. In support of above view, preliminary tests showed that oil production was sensitive to blockade of the flow of air and largely enhanced.
Figure 1:Learning from empirically designed pilot plant run in Philippine. A. Location of the model pilot plant run for 5 years in Manila, Philippines. B. Proposed concept of carbon circulation mimicking the photosynthetic fixation. C. A novel photosynthesis-inspired carbon-fixation model concomitantly releasing oxygen as byproduct. D. Schematic flows of fuel production processes via conversion (transformation) of atmospheric CO2 in the presence of activated catalysts. E. Spraying apparatus and reaction tank installed in the pilot plant. F. Transparent 1/1000-scaled model reaction tank for laboratory tests.
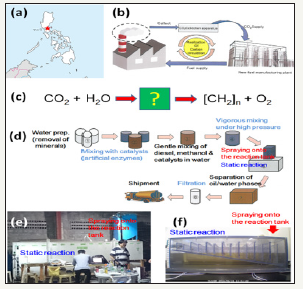
Figure 2:Chemical properties of recovered fuel. A. Gas chromatograms of original and recovered fuels. B. Differentially displayed changes in chain length of hydrocarbons. C. Density of fuels compared. D. Elemental compositions in original and recovered fuels.
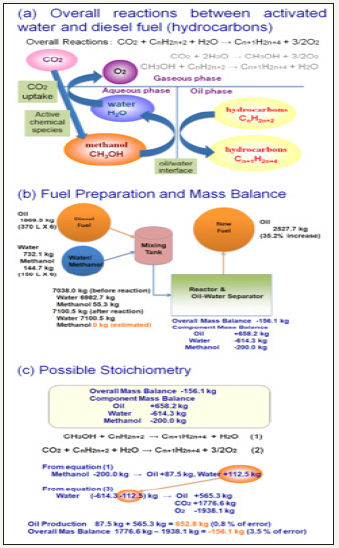
Based on the repeatedly performed mass-balance analyses for the model pilot plants operated in/around Manila, the estimated contribution of the reaction (Figure 3) in which CO2 is the sole carbon source for elongation of hydrocarbon chains (addition of CH2), we have estimated that de novo production of 1lt of fuel is equivalent to capturing of 2.81t of CO2, suggesting that newly recovered diesel can be considered as a novel type of fuel converted (transformed) by addition of atmospheric CO2 in the presence of activated catalysts. New diesel fuel can be thus considered “carbon neutral” by analogy to biofuels produced via capturing atmospheric CO2 through natural photosynthesis, thus, this process could be viewed as a novel artificial photosynthesis corresponding to the dark reactions.
Figure 3:Proposed overall equations and stoichiometry for the catalyzed CO2 uptake and fixation into organic molecules focusing on the roles of methanol, CO2, and original hydrocarbons, as the priming agent, major carbon source, and the template molecules. A. Overall reactions consisted of two sub-reactions occurring in the interfaces among gaseous, aquatic and oil phases in the finely mixed reaction system. B. Mass balance of the model system. C. Possible stoichiometric explanations..
Likely Roles of Redox Reactions Catalyzed by Catalyst Mixture
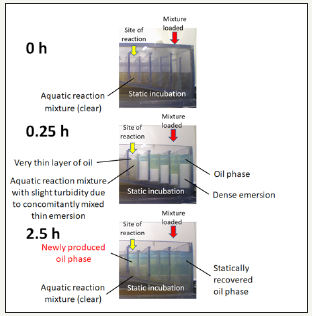
As the aqueous media used for refinement of fuels, catalysts (calcium-rich natural zeolite mixture with supplementation of plant enzymes) were suspended, these catalyst mixtures provided by Chubu enzyme Co. Ltd (Aichi, Japan) could be attributed to the phenomena of interest. Notably, water suspended with these calcium-rich zeolite mixtures drastically elevated the level of superoxide anion radical suggesting the continuous production of radical species is catalyzed. Detection of superoxide was performed with chemiluminescence using Cypridina luciferin analog as the superoxide-specific probe according to the protocols used for enzymatic production of superoxide in the aqueous system [3]. The pilot plant empirically designed for diesel fuel refinement was scaled down by 1/1000 (1/10 width, 1/10 height, 1/10 depth) with transparent acryl models (Figure 1), so that ongoing changes in the volume could be visualized. In this model, catalytic water with suspended catalyst mixture (actively producing superoxide, data not shown) was loaded. Following the high-pressure injection of well-mixed oil/methanol/diesel (prepared with the ratio proportional to the original protocol shown in Figure 3, increase in the oil phase could be initiated at the compartment having thin layer of oil low-density emersion mixture and atmospheric phase meet (Figure 4). Interestingly, the level of superoxide showed tendency to be elevated following injection of fuel mixture coinciding the increase of oil.
Figure 4:Identification of the site of reaction using 1/1000-scalled transparent model mimicking the phenomenon observed in the pilot plant. Reaction set-up prior to (top), immediately after (middle), and after (bottom) the injection of oil/methanol/catalytic water mixture onto the aquatic reaction mixture containing methanol and catalytic water were continuously monitored.
Simplified Model and Possible Mechanism for Absorbing of Carbon (From the CO2 in the Air) to the Hydrocarbons
Figure 5:ISimplified system for monitoring the involvement of CO2 and water during the catalytic water-assisted synthesis of hydrocarbons A. Laboratory-scale model experiments for stoichiometric analysis. B. Synergistic roles of catalytic aqueous mixture and oil for enhanced uptake of CO2 from the air phase. C. Continuous monitoring of CO2 uptake by dodecane-containing mixture. Composition of reaction mixture: 100mL water, 150mL diesel or dodecane, 2.5mg Citric Acid/100mL water, 0.5mL MeOH/100mL dH2O. For model experiments with diesel, suspension of calcium-rich zeolite (Chubu Enzyme Co. Ltd.) was used as catalyst. For model experiment with dodecane, 20mg human hemoglobin (Hb)/1mL 100Mm Phosphate buffer (pH 7) supplemented with 2.7mg FeSO4*7H2O was used as model redox catalysts.
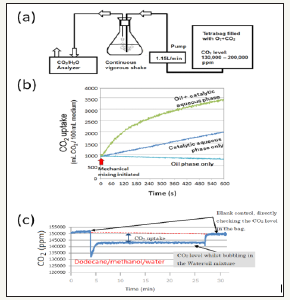
Based on the proposed overall reactions in the pilot plant (Figure 3), we have focused on the utility of CO2 and water as the carbon and hydrogen sources contributing to the formation of hydrocarbons. For assessing the stoichiometry involving CO2 and water, we employed a simplified model which allows continuous monitoring of CO2 and vapor of water flow out by monitoring with CO2/H2O2 analyzer (LI-840A, LI-COR, Lincoln, NE, USA) (Figure 5). We have confirmed that uptake of atmospheric CO2 by liquid phases could be maximized when both the catalytic water and the oil (diesel) co-exist in the system (Figure 5). In this model system, containing either of commercial diesel or a model hydrocarbon (dodecane), uptake of CO2 (Figure 5, the case of dodecane shown) and loss of water were continuously traced. We have evaluated the increase of oil phase accompanying the CO2 uptake by an emulsion with catalyst-suspended water and oil (commercial diesel or dodecane) as summarized in Table 1 & 2. With diesel and calciumrich zeolite, stoichiometry of carbon and water nicely matches the yield of oil while dodecane model experiment employing Hb showed a similar stoichiometric match with lesser extent.
Table 1:Mass-balance in the Diesel/Methanol/Water system.
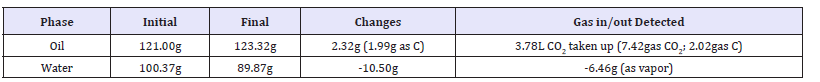
Table 2:Mass-balance in the Dodecane/Methanol/Water system.

Likely Mechanisms CO2 Show High Affinity Towards Hydrocarbons
As shown in the conceptual illustration (Figure 6) representing the movement and conversion of chemical components through the air-oil, oil-water (+methanol), and air-water interfaces within the micro-domains in the water-methanol-oil emersion whipped with air; CO2 in the atmospheric phase enters the phase of oil (thin layer of hydrocarbons) since CO2 show high affinity towards hydrocarbons [3-5]. Note that this type of phenomena can be also observed in most organic solvent even in methanol [6-9], thus CO2 can be statically captured (stored) in and readily released out upon mechanical treatment. Taken together, hydrocarbons (including diesel fuel) and organic solvents function as chemical funnel for capturing and concentrating the CO2.
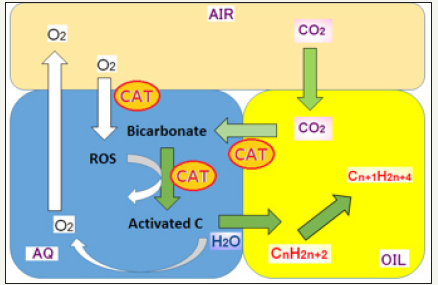
Figure 6:Proposed role of catalysts in the micro-environments consisted of three different phases of materials. CAT: Catalysts; ROS: Reactive Oxygen Species. Illustration provided in the earlier material) presented at international conferences (Kawano T et al. [3] and Uezu k et al. [7,9] University of Kitakyushu International Photosynthesis Industrialization Research Center, 2016)
Enhanced Translocation of CO2
The capacity for maintaining CO2 in the hydrocarbons must be limited depending on the chemical properties of hydrocarbon chosen and conditions given. In fact, diesel fuel exposed to ambient air is likely saturated and thus no further uptake of CO2 can be expected as shown in the model experiments (Figure 5, oil phase only). Therefore, system must be designed to allow translocation of CO2 from oil phase to aqueous phase in the finely mixed oil and aqueous solution. Again, note that capacity for CO2 in the aqueous phase is limited (readily saturated). In nature, plants were evolved to solve this type of problem, by producing catalysts called carbonic anhydrase which catalyzes the conversion of CO2 into bicarbonate (CO2+H2O⇔H++HCO3-). One of the roles for catalysts (calcium-rich natural zeolite mixture with supplementation of plant enzymes) employed in our diesel processing system is enhanced conversion of CO2 into bicarbonate in the aqueous phase, thus allowing continuous flow of oil-aqueous CO2 translocation [5-8].
Synergetic Orchestrated Actions of Catalytic Water Phase and Oil Phase
To prove that radical forming natures (both releasing ROS and C-radicals) in the catalyst-supplemented aqueous phase contribute to enhanced uptake of CO2 by oil phase [9], some laboratory experiments (as one example is mentioned above) were performed and the rate of CO2 uptake was shown to be maximal only when the catalytic water phase and oil phase were finely mixed up.
Radical Formation as Driving Force for Enhanced Uptake of CO2
In the aqueous phase, catalysts may play two distinct roles, namely, [2] generation of reactive oxygen species (ROS) such as superoxide anion radical followed by release of hydroxyl radical, through interaction with dissolved molecular oxygen taken up from the air phase, and [1] generation of carbon-derived radical species coupled to the reactions with ROS. The resultant carbon-derived radical species re-enters the oil phase and react with hydrocarbons. These processes might be the driving force required for creating capacity for further (continuous) CO2 uptake in the water and oil phases and for translocation within the emersion complex. At present, model for several putative carbon-derived radical species are under intensive research activity and the relationship between the radical formation and carbon-fixation (materialized as increase in oil) are studied (unpublished results and hypothetic models) [10].
Acknowledgement
We acknowledge the organizing and scientific committee of the international symposium on Plant Signaling and Behavior 2015 held in Paris for kindly offering the chance to report our idea and key primitive data in front of international audience for the first time dating 29th June 2015 which may crucial for showing the priority of the research. Further confirmations followed as presented (June-July 2017).
References
- Nakamura A, Hara Y, Kawano T (2017) Dewatering and extraction of hydrophilic solutes and essential oils from cryo-preserved lemon peels using liquefied dimethyl ether. Solvent Extraction Research and Development, Japan 24(1): 37-45.
- Comparini D, Uezu K, Kawano T (2015) Monitoring of sonochemically released carbon dioxide from a methanol-water system. Solvent Extraction Research and Development, Japan 22(2): 215-221.
- Kawano T, Kawano N, Hosoya H, Lapeyrie F (2001) Fungal auxin antagonist hypaphorine competitively inhibits indole-3-acetic aciddependent superoxide generation by horseradish peroxidase. Biochem Biophys Res Commun 288(3): 546-551.
- Uemura (2013) Analysis of molecular dynamics of CO2/oil dissolution phenomena in enhanced oil recovery. J Comput Chem Jpn 12(4): 222- 229.
- Hara Y, Kikuchi A, Noriyasu A, Furukawa H, Takaichi H, et al. (2016) Batch extraction of oil from rice bran with liquefied low temperature dimethyl ether. Solvent Extraction Research and Development, Japan 23(1): 87-99.
- Kawano T (2015) Introduction to natural, artificial, and hybrid photosynthesis researches. Plant Signaling and Behavior, Paris, France.
- Uezu K, Kawano T, Comparini D, Uchimura O (2015) Chemical engineering approach for artificial photosynthesis system. PSB2015: Plant Signaling and Behavior, Paris, France.
- University of Kitakyushu International Photosynthesis Industrialization Research Center (2016) Artificial fuel production system mimicking plant photosynthesis: Poster presentation at G7 Kitakyushu energy ministerial meeting.
- Uezu K, Kawano T, Uchimura O (2017) Prediction of the reaction scheme for novel artificial photosynthesis system by the chemical engineering approach. 5th International Symposium on Plant Signaling and Behavior Matsue, Japan.
- Kawano T, Comparini D, Uchimura O, Uezu K (2017) Natural and artificial photosynthesis models for capturing carbon dioxide into fuels. 5th International Symposium on Plant Signaling and Behavior Matsue, Japan.
© 2018 Tomonori Kawano. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






