- Submissions

Full Text
Progress in Petrochemical Science
Kinetics and Mechanism of the Process of Obtaining Liquid Products from the Catalytic Cracking Gases
RP Jafarov*, AA Kasimov, Kh B Piriyeva and SA Jamalova
Mamedaliev Institute of Petrochemical Processes, Azerbaijan National Academy of Sciences, Azerbaijan
*Corresponding author: RP Jafarov, Mamedaliev Institute of Petrochemical Processes, Azerbaijan National Academy of Sciences, Baku, Azerbaijan
Submission: January 19, 2018; Published: February 27, 2018

ISSN 2637-8035Volume1 Issue2
Introduction
The goal of the kinetic study of the process along with chemical and physicochemical methods is to establish the most probable mechanism of the reaction and to build on its basis an adequate mathematical model of kinetics. In addition to the purely theoretical significance of the study of the regularities of the reaction, the creation of an adequate mathematical model of the reaction is a prerequisite for the successful application of mathematical modeling techniques for the optimization and scaling of chemical processes that make it possible to study the reaction in the laboratory in the shortest possible time and proceed to its industrial implementation.
Experimental Part
Investigation of the kinetic regularities of the process of obtaining liquid products from catalytic cracking gases was carried out in a flow-type laboratory reactor with a fixed bed of zeolite containing catalyst, modified with metals of Groups VI and VII, in the temperature range 533K-693K, at contact times 0-25sec. The reaction was carried out under isothermal conditions. The temperature in the reactor was controlled by a thermocouple. The feedstock was fed via a microdoser. The reaction was monitored by analyzing a sample of the reaction mixture on an LXM-80 chromatograph. The contact gas was sampled 30minutes after the start of operation, when the system entered a stable mode of continuous conversion of the feedstock. Analysis of the contact gas allows us to say that there are no oxygen-containing compounds. The influence of the reaction conditions on the formation of products were studied. This allowed us to reveal the patterns and suggest a probable mechanism on the basis of which stoichiometric relationships describing individual routes of the reaction were compiled.
To create a kinetic model of the reaction in the general case, it is necessary to describe the nature of the change in each component involved in the reaction. The change in the concentration of a substance in the reacting system takes into account the participation of this substance in all reactions. Therefore, for each substance, we can write an equation:
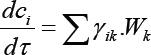
c1,c2,c3c4, c5, c6, c7, c8, c9, c10, c11- are respectively the of propane, propylene, hydrogen, isobutane, isobutylene, ethylene, methane, liquid products (C8H18, C7H16,C6H14), Wk -the rate of the k-th reaction, yik-stoichiometric coefficient of the i-th substance in the k-th reaction. Based on the probable scheme of the reaction mechanism, is compiled a system of differential equations which describes the changes in the concentrations of the initial substances and the reaction products in time. The kinetic constants (ki and ni) of the differential equations (1) were estimated by a modified random search method with automatic step selection. Representing the dependence of the reaction rate constants on temperature in the form of the Arrhenius equation, we find the activation energy (Ei) and the pre-exponential factors (K0). Orders of the reactions are also defined.
The adequacy of the model was checked by minimizing the sum of the squares of the difference between the experimental and calculated values by the formula:
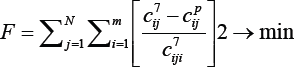
j=1... N-total number of experiments, i=1 ... .m-number of components. The results of the calculation on the PC showed good convergence of the experimental and calculated data. The discrepancies in the initial and final products did not exceed 5-7%. This gives grounds to say that the developed kinetic model of the process of obtaining liquid products from catalytic cracking gases adequately describes the experimental data. The developed kinetic model can be adopted as a basis for designing an experimental industrial reactor operating in an adiabatic regime.
Conclusion
The influence of the reaction conditions on the formation of liquid products were studied, whic made it possible to identify patterns and suggest a probable mechanism. Based on the law of acting masses is developed a kinetic model, which expressed by a system of differential equations. Calculation of the kinetic constants of the differential equations was performed by a modified random search method with automatic step selection. The kinetic model is developed based on the law of acting masses, which is expressed by a system of differential equations.
References
- Slinko MQ (2000) Kinetic and Catalysis 41(6): P.633.
- Abilov AQ, Veliyeva FM, Aliyev FT (1987) Package of applied programs. Estimation of the kinetic parameters of many fixed stationary catalytic reactions. QOShSFAP SSSR. Reg. N050880000906.
- Ostrovskiy QM (1996) Modeling and optimization of catalytic processes. Moscow, Nauka.
- Ioffe II, Pismen LM (1996) Modeling and optimization of catalytic processes. Moscow, Nauka.
© 2018 RP Jafarov, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






