- Submissions

Full Text
Orthopedic Research Online Journal
Parosteal Lipoma of the Femur Presenting with Impending Fracture
Awdhah A Al-Samhan1*, Rola H Ali2, Abdullah A Alfaraj1, Islam M Eldesouky1, Hatem A Shaker1, Magdy M Abdel-Motaal1
1Department of Orthopedic Surgery, Al-Razi Orthopedic Hospital, Kuwait
2Department of Pathology, College of Medicine, Kuwait University, Kuwait
*Corresponding author:Awdhah A Al-Samhan, Department of Orthopedic Surgery, Al-Razi Orthopedic Hospital, Kuwait City, Kuwait
Submission: December 15, 2023;Published: January 04, 2024

ISSN: 2576-8875 Volume10 Issue4
Abstract
Background: Parosteal lipoma is a rare benign tumor of mature adipose tissue located on the surface of bone firmly adherent to the periosteum. It is typically associated with reactive changes in the underlying bone in the form of cortical thickening, erosions, or bowing which may complicate the clinical picture. In this report, we present a case of parosteal lipoma of the femur associated with exuberant bony changes and clinical concern of an impending fracture.
Case presentation: A 33-year-old female presented with right thigh pain and limping. Multimodality imaging revealed a juxtacortical fat-capped exostosis-like lesion located against the upper meta- diaphysis of the femur accompanied by cortical bone protuberances, focal cortical erosion, and an intramedullary lytic component. A needle core biopsy was performed due to concerns regarding the bony reaction but failed to yield diagnostic material. The patient was considered at increased risk of developing a pathological fracture due to the significant functional pain and the radiological findings and underwent an open excisional biopsy and prophylactic fixation of the impending fracture. Finally, pathological evaluation confirmed the diagnosis of parosteal lipoma and the reactive nature of the bony changes.
Conclusion: Parosteal lipoma is a rare benign lesion occurring on the surface of bone which may resemble exostosis or other more aggressive parosteal tumors. Although histopathological evaluation is often regarded as a gold standard, radiology-pathology correlation is mandatory for establishing the correct diagnosis especially on small biopsy samples. Accurate preoperative recognition of parosteal lipoma and distinction from other juxtacortical bone tumors will aid in appropriate surgical planning.
Introduction
Lipomas are benign neoplasms of mature adipose tissue arising in a variety of anatomical locations: subcutaneous, inter- and intra-muscular, tendon sheath, joints, as well as bone [1]. Lipomas related to bone may arise within the medullary cavity (intraosseous lipoma) or on the surface of the bone (parosteal or juxtacortical lipoma) [2]. Parosteal lipomas are rare, constituting 0.3% of all lipomas, most frequently attached to long bones in middle aged adults, with the femur and radius being the most common locations [2-5]. Despite its intimate relationship with the periosteum, this does not necessarily imply a definite origin from periosteal tissue [6].
Since its original description by Seering in 1836 in the German literature, various reports and reviews of parosteal lipoma have been published. It typically presents as a slowly growing painless swelling [2,7,8] and, if sufficiently large, may impinge on adjacent structures leading to motor and sensory neurologic deficits, with the posterior interosseous nerve in the forearm being the most commonly affected [9-11]. Radiographically, it is characterized by a radiolucent mass surrounding cortical bony protuberances. Findings on multimodality imaging are generally pathognomonic and may obviate the need for biopsy [5]. Yet due to its rarity, juxtacortical location, and accompanying bone alterations, parosteal lipoma may occasionally pose diagnostic difficulties. Awareness of this entity and knowledge of the radiological findings are therefore important to avoid confusion with more aggressive juxtacortical mimics.
Herein we present a case of a 33-year-old female with a parosteal lipoma in the upper meta-diaphysis of femur who was deemed to be at high risk of developing a pathological fracture requiring prophylactic intramedullary nailing.
Case Presentation
A 33-year-old woman presented to the outpatient orthopedic clinic with pain when walking and limping of three years duration. The pain was localized to the right thigh and was dull and aching in quality. There was no recent history of physical injury, fractures, or bone tumors, and no other significant medical or surgical history. On physical examination, the patient walked with a mild antalgic gait trying to avoid pressure on the right lower limb. On inspection, no deformity, muscle atrophy or lumps were noticed. Skin was normal with no dilated vessels, discoloration or evidence of vascular compromise. The anterior upper part of the thigh was tender on superficial palpation but no mass could be felt. Active and passive range of motion of the hip and knee were intact and painfree. There was no neurological deficit.
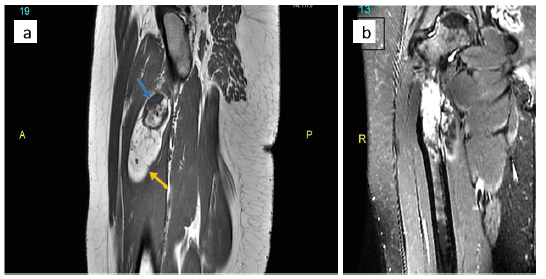
Plain radiographs of the right thigh showed a broad-based exostosis-like projection arising from the medial aspect of the proximal femoral meta-diaphysis, with an overlying well-defined oval-shaped radiolucent component (Figure 1a & 1b). This was associated with a periosteal reaction and an intramedullary lytic area showing dense striations. Computed Tomography (CT) confirmed the presence of a cortical exostosis capped by a soft tissue component of fat density that contained tiny dots of calcification, along with a mildly expansile intramedullary hypodense area (Figure 2a & 2b). On Magnetic Resonance Imaging (MRI), the lesion was inseparable from the cortex protruding from the anteromedial side of the meta-diaphysis into the vastus intermedius. It was composed of an exophytic osseous component with excrescences (5.4 x 3cm) and an overlying well-circumscribed fatty component extending inferiorly (9.0 x 2.6cm), with both components showing signal characteristics that paralleled their respective counterpart tissues (Figure 3a & 3b). No medullary continuity was seen between the exophytic bone and underlying native bone. Additionally, the medulla was expanded by a heterogeneous-signal lesion showing irregular signal-void striations and bright areas on T1-weighted images displaying marginal enhancement post- contrast. However, there was no evidence of infiltration into the surrounding muscle or compression of adjacent neurovascular structures.
Figure 1:Plain radiographs of right femur: (a) anteroposterior view and (b) lateral view. A broad-based bony exostosis (blue arrow) capped by a radiolucent lesion (yellow arrow) is seen on the medial aspect of the proximal meta-diaphysis.
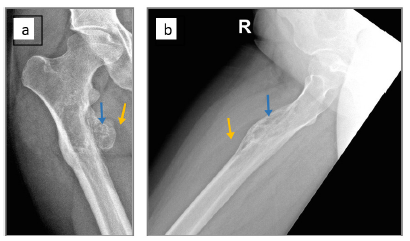
Figure 2:CT images of right femur. (a) Coronal cut showing a proximal meta-diaphyseal cortical exostosis (blue arrow) surrounded by a lucency (yellow arrow). (b) Axial cut showing the soft tissue component of fat density (yellow arrow) with calcified dots.
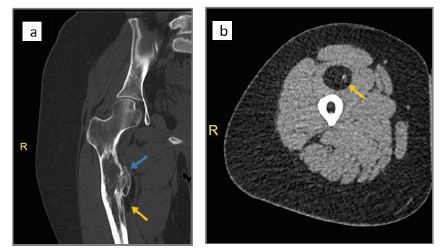
Figure 3:MRI images of right femur. (a) T1-weighted sagittal image demonstrating hyperintense signal of fat in the lesion (yellow arrow) and low signal intensity in the exophytic bone (blue arrow). (b) Coronal Short-Tau Inversion Recovery (STIR) T2 showing suppressed signal in the fatty portion and hyperintensity in the underlying medullary cavity.
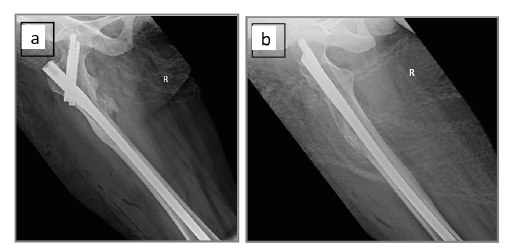
A needle core biopsy was initially performed but yielded nondiagnostic material, hence an open excisional biopsy was done. Intraoperatively, the fatty mass was seen firmly attached to the surface of the femur requiring full dissection to release it from the underlying periosteum (Figure 4a & 4b). Additionally, the exophytic bony portion was completely chiseled from the bone surface and the intramedullary lesion removed by curettage. The resected lipoma and bone were sent to the pathology lab (Figure 4c). Intramedullary nail fixation was performed as a prophylactic measure due to the predicted high risk of a pathological fracture in view of the significant functional pain and the radiological findings (Figure 5a & 5b).
Figure 4:Macroscopic appearance of the parosteal lipoma. (a) Intraoperatively the lipoma was firmly attached to the femoral bone. (b) The lipomatous portion after dissection. (c) The pathological specimen, after inking the surgical margins and slicing it, revealing homogeneous fatty tissue with scattered white foci (red arrow) corresponding to osseous elements.
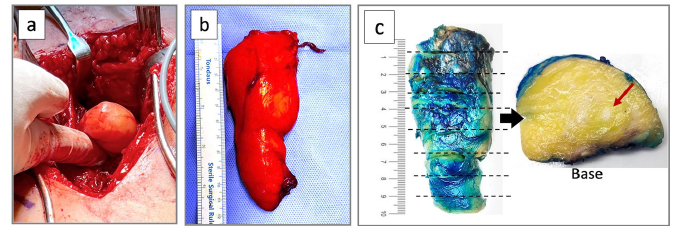
Figure 5:Postoperative films showing the intramedullary nail fixation: (a) anteroposterior view, and (b) lateral view.
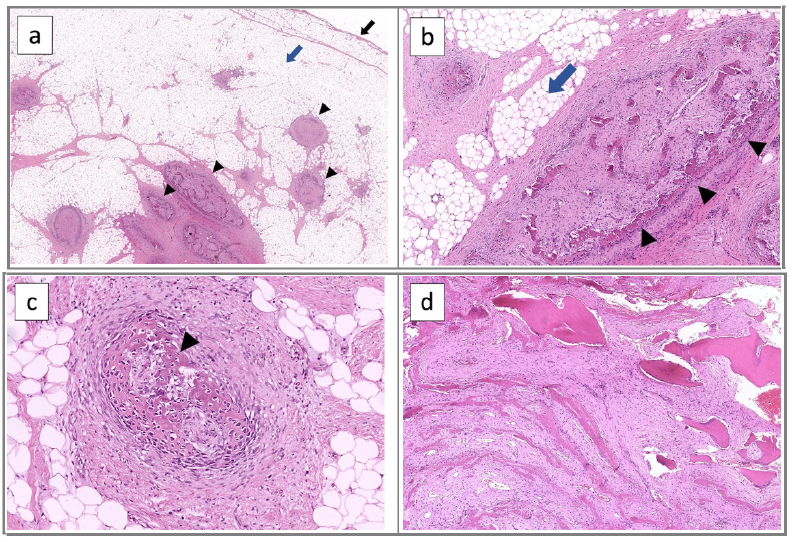
Gross pathological inspection of the fatty component showed an ovoid mass of uniformly yellow adipose tissue measuring 10 x 4 x 3cm, covered by a delicate fibrous membrane (Figure 4c). The broad-based deep surface of the lipoma was irregular and ragged indicating its firm adherence to the bone. On cut section, the yellow adipose tissue was homogeneous with no hemorrhage or necrosis but with scattered foci of white firm tissue. Microscopic examination showed mature adipose tissue with thin bands of fibrovascular septa consistent with lipoma (Figure 6a-6c). Scattered within the substance of the lipoma were islands of mesenchymal tissue merging with spicules of reactive woven bone, corresponding to the white foci identified grossly and tiny calcifications on CT. No hyaline cartilaginous tissue was identified. In view of the juxtacortical location and adherence to the periosteum, the diagnosis of parosteal lipoma was confirmed. The curettage specimen showed reactive changes consisting of thickened cortical along with new woven bone formation in a fibroblastic stroma (Figure 6d).
Figure 6:Histopathology of parosteal lipoma. (a) Overview showing the outer surface of the lipoma (black arrow) with underlying mature fat (blue arrow) containing foci of metaplastic bone (arrowheads), x1 mag. (b) Higher magnification of the fat (blue arrow) and metaplastic bone spicules (arrowheads) merging with mesenchymal tissue, x4 mag. (c) Bone (arrowhead) forming directly from mesenchyme (intramembranous ossification), x10 mag. (d) The bone specimen showing florid reactive bone formation with entrapment of native lamellar bone. (hematoxylin and eosin stain)
Post-operatively, the patient was pain-free and satisfied with the result. A follow up appointment at 14 weeks revealed normal range of movements at the knee and hip and full weight bearing.
Discussion
Parosteal lipoma is a neoplasm of mature adipose tissue this is histologically identical to conventional lipoma and thus lacking unique histological characteristics. What distinguishes parosteal from other lipomas is the intimate relationship with bone, highlighting the importance of radiological-pathological correlation for establishing the diagnosis. Parosteal lipoma most commonly arises against long bones particularly along the femur and radius [5], although it has been reported throughout the skeleton including unique locations such as rib [12], clavicle [13], scapula [14], skull [15], mandible [16], fibula [17], foot [18], and hand [19].
Parosteal lipomas are typically associated with reactive bony changes in the underlying bone, in the form of cortical excrescences, bowing of bone or cortical erosion, in addition to metaplastic osseous/chondroid elements within the fatty substance itself (hence, the pathological designation of “osteolipoma” or “ossifying lipoma”) [4,5,20]. Such heterotopic elements within the fat are not a consistent feature though and may represent multidirectional differentiation of pluripotent mesenchymal cells [21]. Of note, osteolipoma is a histologic variant of lipoma that may occur anywhere independent of bone and is not synonymous with parosteal lipoma [22]. Miller et al. [4] classified parosteal lipoma into four types: 1) those lacking a bony reaction, 2) pedunculated exostosis- like, with cortical bony projections extending into the lipoma, 3) broad-based sessile exostosis-like, and 4) lipomas with patchy bone/chondroid elements throughout the fat. Unlike exostosis (osteochondroma), however, the bone excrescences in parosteal lipoma lack a cartilaginous cap and medullary continuity with the underlying native bone. The current case did show cortical excrescences as well as scattered osseous elements within the fat that were unconnected to the cortex.
The radiological picture of parosteal lipoma may be influenced by the quality and degree of the bony reaction and the histologic composition of the fatty lesion including the amount of fat, bone, cartilage and fibrous tissue [23]. The bony reaction is usually confined to the cortex, yet it may be exuberant or resemble a “sunburst” periosteal reaction of more aggressive bone tumors [24]. In our case, the exuberant reactive bone also involved the medullary cavity. On the other hand, the lipoma itself may show heterogeneous contrast enhancement and septations raising suspicion for well-differentiated liposarcoma [25]. Histopathology examination and close radiological- pathological correlation are warranted in such circumstances.
To sum up, parosteal lipoma is a rare benign tumor that shows characteristic radiographic, CT and MR imaging features enabling correct preoperative diagnosis. Complete surgical removal of the periosteal bone component along with the lipoma is the treatment of choice. Awareness of this entity among orthopedic surgeons, radiologists and pathologists is needed to avoid misinterpretation as an aggressive juxtacortical bone tumor and overtreatment particularly in the presence of exuberant bony changes.
Data Availability
All data generated during this study are included in this article.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Patient Consent
Informed consent has been granted from the patient for the use of her clinical information and images in this manuscript.
References
- Kransdorf MJ, Moser Jr RP, Meis JM, Meyer CA (1991) Fat-containing soft-tissue masses of the extremities. Radiographics 11(1): 81-106.
- Kapukaya A, Subasi M, Dabak N, Ozkul E (2006) Osseous lipoma: eleven new cases and review of the literature. Acta Orthop Belg 72(5): 603-614.
- Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, et (2004) From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics 24(5): 1433-1466.
- Miller MD, Ragsdale BD, Sweet DE (1992) Parosteal lipomas: a new perspective. Pathology 24(3): 132-139.
- Khanna A, Eickstaedt NL, Wenger DE, Broski SM (2023) Multimodality imaging features of parosteal lipomas. Skeletal Radiol 52(9): 1767-1775.
- Power D (1888) Parosteal lipoma, or congenital fatty tumour, connected with the periosteum of the femur. Trans Pathol Soc London 39: 270-272.
- Jones JG, Habermann ET, Dorfman HD (1989) Case report Parosteal ossifying lipoma of femur. Skeletal Radiol 18(7): 537-540.
- Yu JS, Weis L, Becker W (2000) MR imaging of a parosteal lipoma. Clin Imaging 24(1): 15-18.
- Kim HK, Choi YH, Cho YH, Sohn Y, Kim HJ (2006) Intercostal neuralgia caused by a parosteal lipoma of the rib. Ann Thorac Surg 81(5): 1901-1903.
- Tzeng CY, Lee TS, Chen IC (2005) Superficial radial nerve compression caused by a parosteal lipoma of proximal radius: a case report. Hand Surg 10(2-3): 293-296.
- Nishida J, Shimamura T, Ehara S, Shiraishi H, Sato T, et (1998) Posterior interosseous nerve palsy caused by parosteal lipoma of proximal radius. Skeletal Radiol 27(7): 375-379.
- White RZ, Au J, Nguyen T, Sampson MJ (2002) Parosteal lipoma of the rib: A rare condition in an uncommon location. J Med Imaging Radiat Oncol 66(3): 411-413.
- Nie P, Guo J, Xu Y, He Z, Han M, et al. (2017) Parosteal ossifying lipoma of the clavicle: A case report. Mol Clin Oncol 6(3): 419-
- Balani A, Sankhe A, Dedhia T, Bhuta M, Lakhotia N, et al. (2014) Lump on back: a rare case of parosteal lipoma of scapula. Case Rep Radiol 2014:
- Murakami M, Hirai M, Sakakibara T, Yamaki T, Kusuzaki K (2014) Skull parosteal lipoma with reactive hyperostosis: a case report. Neurol Med Chir (Tokyo) 54(4): 314-316.
- Potter J, Richards C, Collin J (2002) Parosteal lipoma of the mandible: A case report and review of the literature. J Oral Maxillofac Pathol 26(1): 129-130.
- Saksobhavivat N, Jaovisidha S, Sirikulchayanonta V, Nartthanarung A (2012) Parosteal ossifying lipoma of the fibula: a case report with contrast-enhanced MR study and a review of the literature. Singapore Med J 53(8): e172-175.
- Ek ETH, Slavin JL, Blackney MC, Powell GJ (2007) Parosteal lipoma associated with an underlying osteochondroma arising from the hallux. Skeletal Radiol 36(7): 689-692.
- Hopkins JD, Rayan GM (1999) Osteolipoma of the hand: a case report. J Okla State Med Assoc 92(11): 535-537.
- Fleming RJ (1962) Parosteal lipoma. Am J Roent 87: 1075-1084.
- Rau T, Soeder S, Olk A, Aigner T (2006) Parosteal lipoma of the thigh with cartilaginous and osseous differentiation: an osteochondrolipoma. Ann Diagn Pathol 10(5): 279-282.
- Demiralp B, Alderete JF, Kose O, Ozcan A, Cicek I, et (2009) Osteolipoma independent of bone tissue: a case report. Cases J 2: 8711.
- Kwee RM, Kwee TC (2019) Calcified or ossified benign soft tissue lesions that may simulate malignancy. Skeletal Radiol 48(12): 1875-1890.
- Asirvatham R, Linjawi T (1994) Ossifying parosteal lipoma with exuberant cortical A case report. Int Orthop 18(1): 55-56.
- Gholamrezanezhad A, Basques K, Kosmas C (2018) Peering beneath the surface: juxtacortical tumors of bone (part II). Clin Imaging 50: 113-122.
© 2024 Awdhah A Al-Samhan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






