- Submissions

Full Text
Orthopedic Research Online Journal
Pediatric Temporomandibular Articulation Ankylosis: OPG and RANKL Gene Expression Analysis
André da Silveira Braune1, Patrícia Cataldo de Felipe Cordeiro2, Letícia Ladeira Bonato3, Ricardo Lopes Cruz1, Jamila Perini1, Valquiria Quinelato2*, Jose de Albuquerque Calasans-Maia2 and Marco Bernardo Cury Fernandes1
1National Institute of Traumatology and Orthopedics, Brazil
2Orthodontics Department, Universidade Federal Fluminense, Brazil
3School of Dentistry, Universidade Federal Fluminense, Brazil
*Corresponding author: Valquiria Quinelato, Orthodontics Department, School of Dentistry, Universidade Federal Fluminense, Brazil
Submission: November 13, 2020;Published: November 25, 2020

ISSN: 2576-8875 Volume7 Issue4
Abstract
Aim: The pilot study aimed to evaluate the association between the presence of Temporomandibular Joint Ankylosis (TMJA) and the expression profile of the RANKL (nuclear kappa-B receptor activator factor/ligand) and OPG (osteoprotegerin) genes.
Methods: Jaw bone tissue samples not committed by ankylosis (jaw coronoid process jaw branch) and ankylotic block were collected from four children with TMJA during ankylosis surgical treatment composed the Test Group and Jaw bone tissue samples (jaw branch, jaw body) were collected from five adults with no history TMJA during jaw surgical treatment (trauma, extraction of mandibular third molar included and jaw dental implant installation) constituted the control group. The total RNA was isolated from osteoblasts using Trizol® The reverse transcription reaction of polymerase chain reaction (RT-PCR) was performed for the synthesis of complementary DNA (cDNA) from 300ng of RNA using the ImProm-II Reverse Transcription System™. Quantitative PCR evaluated OPG/RANKL mRNA expressions.
Results: RANKL gene expression showed reduced ‘levels in the test group compared to the control group (P=0.01). There was no significant difference between the levels of OPG gene expression (P=0.32) as well as the OPG/RANKL ratio (P=0.34) between the groups.
Conclusion: A change in the bone homeostasis from children with temporomandibular joint ankylosis favors the bone synthesis.
Keywords: Temporomandibular joint; Ankyllosis; Gene expression; RANK; OPG
Introduction
Temporomandibular Joint Ankylosis (TMJA) is defined by bone and/or fibrous fusion of joint surfaces associated with limitation of masticatory function, nutritional imbalances and psychosocial changes [1-3]. Additionally, TMJA has a direct influence on craniofacial development due to the impairment of structural growth sites such as the mandibular condyle [4]. Specifically, craniofacial deformities are still related to dental malocclusions [3] and obstruction of airways, resulting in increased risk of recurrent infections and obstructive sleep apnea [5].
TMJA frequently occurs in the first decade of life [6] and severity varies with the degree of structural involvement [7]. The etiology of TMJA is classified as multifactorial, including facial trauma [8], poorly treated otitis [9] and neoplasms [1]. Additionally, molecular, genetic factors related to bone tissue also play an important role in the development of TMJA [7,10,11].
Bone structural integrity is established by bone remodeling processes in which synthesis and resorption occur simultaneously. This tissue homeostasis is mediated by osteoblasts and osteoclasts signaled by various molecular factors [7] such as osteoprotegerin (OPG), the kappa B nuclear factor activating receptor (RANK) and its ligand RANK (RANKL). The osteoblastic lineage regulates osteoclastic activity via the RANK/RANKL/OPG signaling pathway [12]. RANKL is produced by osteoblasts and binds to RANK present in osteoclast precursor cells stimulating differentiation and osteoclastic activity. The interaction between RANKL and its RANK receptor is controlled by osteoprotegerin (OPG), which inhibits the binding of RANKL to RANK, competing with it for the same ligand, and thus acts as a protective factor against bone resorption [7,12].
The biological basis of the etiology of TMJA is still unknown, which limits clinical practice and is commonly directed to multiple surgical treatments from childhood to adulthood [8]. Thus, the identification of genetic biomarkers associated with TMJ ankylosis and bone metabolism is a promising approach for personalized diagnosis and treatment. Currently, temporomandibular disorders and TMJA have already been related to genetic alterations of the RANK/OPG/RANKL triad in local manifestations [13] and in association with other arthropathies [14], confirming the systemic and primordial role of the molecular triad. However, the molecular and genetic mechanism associated with TMJA has not yet been elucidated, as well as genetic factors related to predisposition to ankylosis development in children. In this context, the present study aimed to evaluate the association between the presence of Temporomandibular joint ankylosis (TMJA) and the expression profile of the RANKL and OPG genes.
Materials and Methods
The clinical and laboratory protocol of the pilot study was approved by the Research Ethics Committee (CEP) of the National Institute of Traumatology and Orthopedics Jamil Haddad (INTO), under number 401.481, on September 20, 2013. All included research participants, or their legal representative, agreed and signed the informed consent and/or informed consent prior to the conduct of the research.
Sample characterization / surgical protocol for biological material collection
Eleven Children diagnosed with TMJ ankylosis attending the Maxillofacial Skull Surgery sector at INTO were evaluated during the period from November 2013 to March 2017. The inclusion criteria of the study were individuals between 8 to 17 years of age requiring surgical treatment of TMJ ankylosis.
Prior tomographic recordings were performed to assess coronary process hypertrophy and true bone fusion in the TMJ ankylosis group. Surgical access was performed through a pre-auricular incision with temporal extension and bone tissue samples not committed by ankylosis (mandibular branch and/or coronoid process) and ankylotic block were collected during surgery treatment of TMJA, coronary process hypertrophy and/or mandibular extractor installetion. Thus, only four Children (n=4) needed for surgical therapy and they were included in this study.
The control group consisted of adult individuals without pathological alterations in the ATM, no history TMJA, who underwent mandibular surgery treatment. Thus, Jawbone tissue samples (jaw branch, jaw body) were collected from five adults during jaw surgical treatment (trauma, extraction of mandibular third molar included and jaw dental implant installation). The health history, identification of past trauma and infections prior to the manifestation of TMJ ankylosis were evaluated through anamnesis form and dental clinical examination.
Processing of biological samples
All tissue samples collected were sent to the Jamil Haddad National Institute of Traumatology and Orthopedics (INTO) Clinical Research Center for immediate processing by washing with sodium phosphate buffer (PBS), mechanical grinding. The Bone fragments were subsequently digested with 1% collagenase (Sigma-Aldrich, St. Louis, MO) for 2 hours at 37 °C. Harvested cells were centrifuged at 300g for 10 minutes and resuspended in Dulbecco's modified essential medium (low glucose DMEM, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum selected by batch (FBS, Gibco). A total of 1.0 x 105 cells were plated in 75cm2 flasks and allowed to grow to 70% confluence in a humidified atmosphere of 5% CO2 and 37 °C. Osteoblasts were subsequently harvested by enzymatic digestion with 0.125% trypsin and 0.78mM EDTA (both from Sigma-Aldrich) and expanded to passage.
RNA extraction and gene expression analysis
Total RNA was isolated from osteoblasts (1x106) using Trizol® reagent (Invitrogen™, Life Technologies, NY, USA) according to the manufacturer's protocol. Samples were treated with DNase to digest genomic DNA using DNase DNA-free (Ambion por Invitrogen™). RNA integrity was confirmed on 1.2% agarose gel electrophoresis stained by electrophoresiswith Nucleic Acid Gel Stain® (Invitrogen™). RNA purity was confirmed with spectrophotometric absorbance at a ratio of 260/280 Nanodrop® 1000, Thermo Scientific, Wilmington, DE, EUA). Only RNA samples with a 260/280 ratio above 1.7 were included in this study. The samples were stored at -80 ºC and properly cataloged.
The reverse transcription reaction of polymerase chain reaction (RT-PCR) was performed for the synthesis of complementary DNA (cDNA) from 300ng of RNA using the ImProm-II Reverse Transcription System™ (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer's protocol. Quantitative polymerase chain reaction (qPCR) was performed on the Line Gene 9660 (Bioer Technology, Binjiang, China) using the SYBR Green Master Mix rapid detection system (Applied Biosystems, Foster City, CA, USA) with 1.5µl cDNA in each reaction. The qPCR was performed under the following conditions: 95 °C for 10 minutes, followed by 40 cycles of denaturation and polymerization (95 °C for 15 seconds, 60 °C for 1 minute and 72 °C for 30 seconds) plus the Melting curve for analysis of SYBR fluorescence pattern in reactions (95 °C for 15 seconds, 60 °C for 30 seconds e 95 °C for 15 seconds).
The specific primers for the OPG and RANKL genes were made based on BLAST data. The values were normalized in relation to the constitutive expression of β-actin. The Livak method (2-ΔΔCT) was used to determine the relative quantification of RANKL/OPG expression. Two series of experiments were performed for each tissue sample to ensure reproducibility and all reactions were performed in duplicate. Primer Sequences used for qPCR expression analysis for the genes were: OPG (forward 5'-AGGAGCTGCAGTACGTCAAG-3' and reverse 5'-TCTGGGGTTCCAGCTTGC-3'), RANKL (forward 5’-GCAGAGAAAGCGATGGTGGA-3' and reverse 5'-GGAACCAGATGGGATGTCGG-3'), β-actina (forward5'-AATTACGAGCTGCGTGTGG-3' and reverse 5'-AGAGCGCAGGTAGGATAGCA-3').
Statistical analysis
The Shapiro-Wilk test was used to evaluate the distribution between the variables. The Kruskal-Wallis test was used for comparison between groups, including gene expression analysis after calculation by the 2-ΔΔCT method. P values <0.05 were considered statistically significant. Statistical analyzes were performed using the software Graphpad Prism 7 (GraphPad Software, Inc. La Jolla, CA, EUA) and Excel.
Results
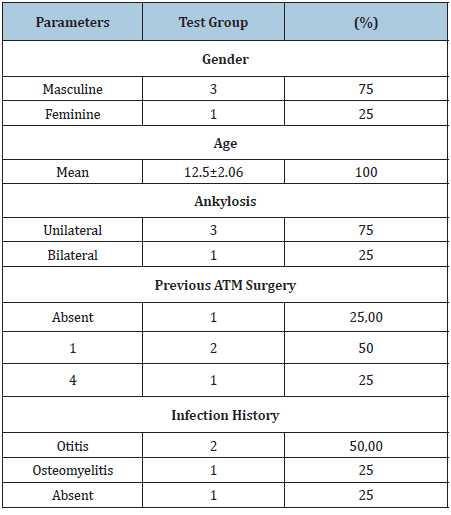
The Test group was consisted of 4 children (n=4) the average age of the participants was 12.5±2.06 years and 3 (75.00%) subjects reported a history of previous infection in the neonatal period in the health history and dental clinical evaluation (Table 1). The average age of the control group was 34.66±13.02 years, gender was three (60%) men and two (40%) women (Table 1).
Table 1: Test group clinical information.
The expression level values of the OPG and RANKL genes in the test (ankylotic block and Bone) and control groups did not show normal distribution pattern by the Shapiro-Wilk test. Thus, Kruskal-Wallis test was used at a significance level of 0.05 to compare the levels of mRNA of the OPG and RANKL genes between the groups (Table 2).
Table 2: Mean individual values of gene expression, OPG and RANKL, considering all samples in the studied groups (Livak method).
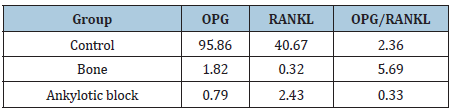
The gene expression OPG/RANKL ratio showed no significant differences among the control, ankylotic block and Bone groups (p=0.72) (Figure 1) as well as the OPG mRNA gene expression was compared between the groups did not show significant difference (P=0.50) (Figure 2).
Figure 1: Analysis of the ratio of OPG and RANKL gene expression comparing between control, ankylotic block and bone groups.

Figure 2: Comparison of OPG gene mRNA expression between groups.

There was no significant difference between control and Ankylotic block groups (P=0.44). Comparison between control and Bone groups showed significant differences in RANKL mRNA gene expression levels (P=0.01) (Figure 3).
Figure 3: Messenger RNA expression levels of RANKL gene. Comparation among the groups.

Discussion
The biological basis of Temporomandibular joint ankylosis (TMJA) has not yet been completely elucidated, however it is known that there is a deregulation of the bone homeostasis process and maintenance of the normal anatomical architecture of the TMJ, culminating in the formation of a continuous bone block characteristic of TMJA [13]. Bone homeostasis is influenced by individual factors and is directly related to the function of the RANK/RANKL/OPG glycoprotein molecular triad [9,13].
Temporomandibular joint ankylosis (TMJA) represents a challenge in clinical practice [15] with high relapse rates and the need for additional surgical interventions throughout the craniofacial growth process [1]. In the present study, the mean age of the test group was 12.5±2.06 years and three children (75.00%) underwent previous surgery to treat TMJA throughout childhood.
The aim of this study was to evaluate the association between the presence of TMJA and the RANKL and OPG mRNA levels. Thus, a low RANKL expression was observed in the test group compared to control group (p=0.01), suggesting that the differentiated repair pattern does not occur only in areas of the ankylotic block, which highlights the systemic alteration of the pattern of remodeling that favors the bone synthesis. There was no significant difference between control and Ankylotic block groups (P=0.44).
The involvement of this triad (RANK/RANKL/OPG) in the pathogenesis of TMJA has been previously investigated [13] and a reduced RANKL/OPG ratio was observed in ankylotic block samples compared with the control group, suggesting that osteoclast deficiency may be an important factor for the development of Temporomandibular joint ankylosis. When osteoclast stimulation is impaired and resorptive capacity is deficient, the process of bone remodeling will be impeded and lead to a progressive increase in bone density [13]. However, the present study did not show significant differences in gene expression of the gene expression OPG/RANKL ratio showed no significant differences among the groups (p=0.72).
The specific role of genes in the etiology of TMJA is still unknown, but in association with other etiological factors may favor the development of the disease. Local trauma and poorly treated infections are reported as major causes of childhood TMJA [16]. In the present sample, three (75.00%) participants in the experimental group had a previous history of infection. However, the high frequency of such factors in childhood is not directly related to the rare manifestation of the disease in the population. Structural imbalance occurs only in individuals genetically predisposed to bone apposition behaviour [7,10].
The genetic influence as the etiological basis of TMJA has been demonstrated by studies of genetic polymorphisms. The ANKH gene polymorphism was associated with TMJA [10]. Additionally, OPG gene polymorphism (rs2073618) was presented as a potential marker associated with the risk of TMJA manifestation [7]. Specifically, plasma OPG levels were associated with intronic ANKH genetic polymorphisms [17]. The results of these studies strengthen the findings of this pilot study.
Identification of genetic biomarkers for pediatric TMJA is a promising approach for personalized diagnosis and treatment. In the present pilot study, low RANKL expression in test group when compared whit control (p=0.01), signaling a possible change in the bone homeostsis from children with temporomandibular joint ankylosis that favors the bone synthesis. RANKL expression also has been researched in other pediatrics diseases: Type 1 diabetes mellitus (T1DM), Alkaptonuria, Osteogenesis imperfecta, 21-Hydroxylase deficiency and Prader-Willi syndrome. All diseases presented RANKL upregulation, but the Hemophilia A presented normal or Increased levels of RANKL [18]. Unlike TMJA, these pathologies present reduced bone mass.
However, the need for molecular investigations in the pathogenesis of TMJA is highlighted, elucidating etiological factors, and directing specific therapies. In pediatric individuals, individualized genetic therapeutic approaches promise to reduce relapses and additional surgical interventions throughout the craniofacial growth process.
Despite being a pilot study, this research shows some relevance for evaluating a rare disease with great psycho-social impact on the lives of children with condylar ankylosis. The TMJA affects 1:2173.91 children in a study that evaluated 21.720 children over a one-year period [6].
Limitations
Despite some limitations, such as sample size and composition of the control group, this study has great relevance for evaluating a rare disease with great psycho-social impact on the lives of children with condylar ankylosis.
Conclusion
A change in the bone homeostasis from children with temporomandibular joint ankylosis favors the bone synthesis.
Conflicts of Interest
The authors have none to declare.
Acknowledgement
The authors are gratefully acknowledge the assistance of the Centro de Cirurgia Craniomaxilofacil, Instituto Nacional de Traumatologia e Ortopedia (INTO) and your team. The support from the Clinical Research Center, Instituto Nacional de Traumatologia e Ortopedia (INTO) and the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ) for research assistance.
References
- Vasconcelos BE, Porto GG, Bessa-Nogueira RV, Nascimento MM (2009) Surgical treatment of temporomandibular joint ankylosis: Follow-up of 15 cases and literature review. Med Oral Patol Oral Cir Bucal 14(1): 34-38.
- Long X, Li X, Cheng Y, Yang X, Qin L, et al. (2005) Preservation of disc for treatment of traumatic temporomandibular joint ankylosis. J Oral Maxillofac Surg 63(7): 897-902.
- Cordeiro PF, Quinelato V, Bonato LL, Braune AS, Santos TB, et al. (2018) Artroplastia interposicional para tratamento de anquilose da articulação temporomandibular: Relato de caso pediá Rev Port Estomatol Med Dent Cir Maxilofac 59(1): 54-60.
- Rozanski C, Wood K, Sanati-Mehrizy P, Xu H, Taub PJ (2019) Ankylosis of the temporomandibular joint in pediatric patients. J Craniofac Surg 30(4): 1033-1038.
- Al-Nuumani IK, Bakathir A, Al-Hashmi A, Al-Abri M, Al-Kindi H, et al. (2018) A triad of temporomandibular joint ankylosis, mandibular retrognathia and severe obstructive sleep apnoea: Case report. Sultan Qaboos Univ Med J 18(3): e379-e382.
- Gupta VK, Mehrotha D, Malhotra S, Kumar S, Algarwal GG, et al. (2012) An epidemiological study of temporomandibular joint ankylosis. Natl J Maxillofac Surg 3(1): 25-30.
- Corso PF, Meger MN, Petean IBF, Souza JF, Brancher JA, et al. (2019) Examination of OPG, RANK, RANKL and HIF1A polymorphisms in temporomandibular joint ankylosis patients. J Craniomaxillofac Surg 47(5): 766-770.
- Manganello-Souza LC, Mariani PB (2003) Temporomandibular joint ankylosis: Report of 14 cases. Int J Oral Maxillofac Surg 32(1): 24-29.
- Chidzonga MM (1999) Temporomandibular joint ankylosis: Review of thirty-two cases. Br J Oral Maxillofac Surg 37(2): 123-126.
- Huang B, Takahashi K, Sakata T, Kiso H, Sugai M, et al. (2011) Increased risk of temporomandibular joint closed lock: A case-control study of ankh polymorphisms. PLoS One 6(10): e25503.
- Tsui FW, Tsui HW, Cheng EY, Stone M, Payne U, Reveille JD, et al. (2003) Novel genetic markers in the 59-flanking region of ANKH are associated with ankylosing spondylitis. Arthritis Rheum 48(3): 791-797.
- Otero L, García DA, Wilches-Buitrago L (2016) Expression and presence of OPG and RANKL mRNA and protein in human periodontal ligament with orthodontic force. Gene Regul Syst Bio 10: 15-20.
- He LH, Xiao E, Duan DH, Gan YH, Zhang Y (2015) Osteoclast deficiency contributes to temporomandibular joint ankylosed bone mass. Formation. J Dent Res 94(10): 1392-400.
- Bonato LL, Quinelato V, Borojevic R, Vieira AR, Modesto A, Granjeiro JM, et al. (2017) Haplotypes of the RANK and OPG genes are associated with chronic arthralgia in individuals with and without temporomandibular disorders. Int J Oral Maxillofac Surg 46(9): 1121- 1129.
- Sidebottom AJ (2013) Management of recurrent ankylosis in arthrogryposis: new solutions to a rare problem. Br J Oral Maxillofac Surg 51(3):256-8.
- Kaban LB, Bouchard C, Troulis MJ (2009) A Protocol for management of temporomandibular joint ankylosis in children. J Oral Maxillofac Surg 67(9): 1966-1978.
- Vistoropsky Y, Malkin I, Kobyliansky E, Livshits G (2007) Osteoprotegerin plasma levels are strongly associated with polymorphisms in human homologue of the mouse progressive ankylosis (ankh) gene. Ann Hum Genet 71(Pt 3): 302.
- Brunetti G, D'Amato G, Chiarito M, Tullo A, Colaianni G, Colucci S, Grano M, Faienza FM (2019) An update on the role of RANKL-RANK/osteoprotegerin and WNT‑s‑catenin signaling pathways in pediatric Diseases. World Journal of Pediatrics 15(1): 4-11.
© 2020 Valquiria Quinelato. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






