- Submissions

Full Text
Orthoplastic Surgery & Orthopedic Care International Journal
A Review of Biomaterials for Bone Tissue Regeneration
Shithima Sayed1, Omar Faruq2* and Gunes Uzer2
1Department of Ophthalmology, South Korea
2Department of Mechanical and Biomedical Engineering, USA
*Corresponding author: Omar Faruq, Department of Mechanical and Biomedical Engineering, USA
Submission: July 04, 2022;Published: July 20, 2022
ISSN 2578-0069Volume2 Issue5
Abstract
Bone is one of the critical tissues which undergoes a self-healing and regeneration process during minor damage. The regeneration capabilities of bone may create an interest in the research for encountering the large defects using biomaterials derived scaffold. Scaffold can support the cells growth, proliferation and differentiation that subsequently improves the healing rate. The selection of biomaterials with appropriately designed scaffold is desirable to advance orthopedic surgery and improve patient quality. Different types of biomaterials have been used in repairing the defective bone tissue. Ceramic materials are most promising due to their mimic the native bone tissue. Natural and synthetic polymers have recently been used in bone tissue engineering to improve scaffold performance. Polymers are suitable for sustained the release of drugs and growths factor to influence osteoinduction. Metallic implants are another type of biomaterial that replace the damaged bone or support the fractured bone during the healing process. Appropriate surface coating tailored to the metal improves the bonding between the native bones. Clinically proper biomaterials selection is challenging to support bone growth. In this review article, we highlight the biomaterials frequently applied in bone tissue engineering, and overview their properties in terms of cell attachment, proliferation and differentiation. Moreover, we discuss the current challenges and limitations of biomaterials in orthopedic applications.
Keywords: Regeneration; Biomaterials; Scaffold; Ceramic; Metal; Polymer
Keywords:PCL: Poly(ԑ-Caprolacatone); BCP: Biphasic Calcium Phosphate; PLLA: Poly(L-Lactic Acid); PLGA: Poly(Lactic-Co-Glycolic Acid)
Introduction
Bone is one of the organs in the body which uniquely composed of mineralized hard tissue [1]. It can protect different parts of our body from major damage and minor accident. Bone has unique properties that constantly could be remodeled throughout life and regenerate by itself in response to damage [2]. However, in large-scale damage and critical condition, surgical performance will be needed for the implantation of the bone substitutes to regenerate and repair the bone defects [3]. Advanced research improves the bone substitute quality in biocompatibility, osteo-conduction and osteoinduction. Among the different kinds of bone substitutes, autografts are still the best option for bone repair. However, they lack overall surgical demand for bone regeneration. In addition, harvesting the bone graft is critical for the patient and confronts a postoperative complication [4]. Orthopedic surgeon frequently utilizes allograft and xenograft, but the immune rejection, drug transmission is a major risk for the patient. Researchers currently focus on synthetic bone substitute that it resolves the existing limitation of other bone substitute. Biomaterials-derived synthetic bone substitutes rapidly meet the demand for orthopedic implants and create the best option in the future for surgical applications. Synthetic bone scaffolds explicitly regenerate the bone defect and significantly assists during the remodeling process.
Recent research focuses on improving biomaterials to enhance their physical and mechanical properties, chemical modification to immobilize the growth factor and stem cells and reduce the risk of immune rejection. The progress of biomaterials performance remarkably declines the gap between the synthetic scaffold and other bone graft. Moreover, researchers fabricate unique scaffolds utilizing advanced biomaterials that drastically increase patient quality. The critical issue of scaffold after surgical implantation is immunoreaction with inflammatory cells, long-term immunoreaction may result in rejection the implant materials. Chemically modified biomaterials have recovered the immunoreaction and improved the cell-materials reaction in the physiological system. Furthermore, mechanical stability of bone scaffold also requires for hard tissue repair that could be achieved by the modification of biomaterials. Therefore, the advancement of biomaterials and their modification is crucial for unique scaffold development in bone tissue regeneration. In the current review article, we explore the different types of biomaterials that explicitly play a role in bone regeneration and provide an overview of their function in biological system. Our discussion also highlights the current shortcoming and unmet challenges of biomaterials for the orthopedic application.
Biomaterials for Synthetic Scaffold
Successful implantation of scaffold largely depends on their physical feature and the type of biomaterials used for fabrication. Therefore, different types of biomaterials have been applied for bone tissue engineering [5]. Multiple issues are involved in choosing the biomaterials for orthopedic application, including mechanical and biological properties. The common biomaterials for hard tissue repair are ceramic, polymers, metal, and different composites materials [5,6]. Each type of biomaterials has certain advantages and disadvantages; high performances biomaterials are continuously under development [7] (Table 1).
Table 1:Biomaterials in bone tissue engineering.

Ceramic biomaterials
Ceramic biomaterials are the most common for orthopedic surgery to repair and regenerate hard tissue. Their chemical properties resemble bone matrix that attract researcher utilizing ceramic for bone tissue engineering. They held bone-like characteristics that degrades during the healing process to replace new tissue. Calcium phosphates are the most popular ceramic biomaterials applied in scaffold fabrication, as they are similar to inorganic part of the bone tissue. Different type of calcium phosphates, such as hydroxyapatite and β-tricalcium phosphate, has developed [8]. In contrast to β-tricalcium phosphate, hydroxyapatite shows slow degradation and resorbed in the body. Their appropriate combination plays a significant role in terms of osteoinduction and osteo-conduction. Biphasic Calcium Phosphate (BCP) is composed of hydroxyapatite and β-tricalcium phosphate that has inherent characteristics of osteoconductivity and biocompatibility [9,10]. Calcium phosphate -based scaffold release Ca2+ and PO4-3 that helps to the formation of an appetite layer that can adsorb the osteogenic protein. Ion release behavior also maintains the deposition of ECM component that potentially influence the osteoinduction during the healing process. Further modification of calcium phosphate ceramic biomaterials could be achieved by incorporating Zn, Si, Sr.(28=32). Bioglass is another class of ceramic materials that is highly biocompatible and formations of bone in the surrounding tissue [11]. Rapid degradation of bioglass enhances gene expression, upregulating cellular activities and create a bond with the surrounding bone [11]. The most common bioglass is 45S5 composed of 45wt% SiO2, 25.5wt% CaO, 24.5wt% Na2O and 6.0 wt% P2O5. Compared to calcium phosphate, the bone regeneration capability of bioactive glass undergoes a series of intersurface reaction that subsequently influence the formation of apatite layer on the material surface. In addition, the release of ions such as Ca2+, PO43- , and Si4+ has potentially played a role in osteogenesis. The ion release behavior of bioglass create much more interest to the researcher to modify the bioglass for better performance in osteogenesis. For example, the Sr-incorporation bioglass showed early angiogenesis through altering the inflammatory macrophage phenotype. Lemon et al. [12] developed the bioglass microsphere that has shown significant bone regeneration in in-vivo [12]. In addition, their functional surface and mesoporous formation may attract the tissue engineer to loading the bioactive molecules and drugs for different applications.
Polymeric biomaterials
Polymers are long-chain organic materials exclusively applied in the tissue engineering field. In the fabrication of bone scaffolds, polymers play a significant role of cellular attachment, proliferation, and differentiation [13]. They are broadly classified as natural and synthetic polymers based on their origin. Both have advantages and disadvantages during the tissue regeneration process [14]. Natural polymer collagen provides a desirable homing environment for cells in bone tissue engineering. Collagen contains cell adhesion ligand arginine-glycine-aspartic acid (RGD) that facilitates cell adhesion [12]. Currently, a processed form of collagen, namely gelatin, has been applied for cell attachment and proliferation [15,16]. Alginate derived from algae is another kind of natural polymer which is biocompatible and readily forms cell-friendly hydrogel [17]. It can be easily modified and provide suitable support for cell loading, growth factor, and drug immobilization. Alginate and other polymers combination, provides the desired properties compared to individual polymers [18]. In recent years, silk and soya protein have also exhibited better performance in bone tissue engineering. Silk’s mechanical and chemical properties are suitable for hydroxyapatite formation and guided bone tissue regeneration [19]. On the other hand, soya proteins, due to their availabilities, biodegradability, and inert properties, may influence their application in the tissue engineering field [20]. They are capable of triggering osteoblast cells for collagen synthesis and osteogenic nodule formation. Lee et al. [20] that showed the electrospun scaffold PEO incorporating soya protein significantly improves rat skull’s bone defect [20] (Figure 1).
Figure 1:Polymeric hydrogel loading provide large surface area for cell attachment and migration.
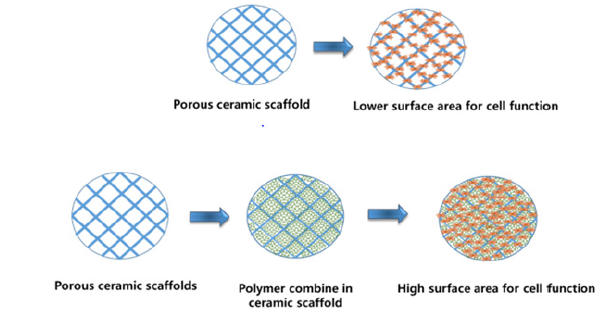
Besides natural polymers, several synthetic polymers have widely used for tissue repair and remodeling. They have more options for modifying of their chemical behavior, physical stabilities, cellular bindings and bioactive compound immobilization [21]. Research could alter the polymers’ properties for cell adhesion by adding RGS peptides or growth factors. In addition, their hydrophobic and hydrophilic nature could be tailored to avoid immunogenicity as well as osteoconductivity. Poly(L-Lactic Acid) (PLLA) is synthetic polymers used to fabricate synthetic scaffold. In the biological system, the hydrolytic degradation of PLLA produce lactic acid in our body. They are often used for coating the metallic implant for better osteointegration. Poly(Lactic-Co-Glycolic Acid) (PLGA) is another synthetic co-polymers of lactic acid and glycolic acid. Their degradation rate depends on ratio of lactic acid and glycolic acid; thus, it can be tunable and adjusted according to their requirement. This can influence their application in sustain release of drugs and growth factors at the site of bone defects. Poly(ԑ- Caprolacatone) (PCL) is widely applied in bone tissue engineering in terms of scaffold fabrication, coating the metallic implant etc. It possesses high mechanical strength that attracts researchers for their application in bone tissue engineering [22]. However, their low degradation behavior and lack of cell attachment limit their application in the orthopedic application. Currently, polydopamine coating, and pepetide incorporation have been utilized in PCLbased biomaterials for bone tissue regeneration [23].
Metallic biomaterials
Metallic biomaterials are widely applied in dental and orthopedic surgery to replace the defect bone and support during the healing. Currently, a metallic implant creates a much more attractive in spinal implants, hip replacements, and long bone fracture repair. Since the mechanically metallic implant is suitable for hard tissue repair, their poor ossteointegration limits their performance in orthopedic surgery. Researches focus on metallic implant surface properties and tailor their surface with bioactive coating for ossteointegration and cellular function. Among the different kinds of metals, Titanium mostly utilized in orthopedic surgery. They are bioinert and in an optimal condition, capable of bonding formation with the surrounding bone. [24] Current research explores the modification of Ti to form the Ti-alloy that showed better cytocompatibility and physical performance compared to other metallic implants. For example, Titaniumaluminum- vanadium alloys have better characteristics in contrast to pure titanium. The significant drawbacks of titanium and its alloys are no degradability in the biological environment; thus, it should be removed after the healing. The postoperative removal of Ti creates further discomfort and damage to surgical site. Mgderived biomaterials are susceptible to biodegradation of their corrosion attributes in a physiological process. The degradation behavior of Mg overcomes the postoperative removal of the metallic implant and provide a space for replacing the new bone [25].
Mg and their alloys are osteoconductive and influences cell attachment and bone growth. The major drawback of Mg alloys increases the local pH through their rapid degradation which limits their application in orthopedic surgery. Current research efforts to reduce their degradation by surface coating. The coating layer should be non-toxic and degrade in a sustainable manner. Among the different coating layers, Ca-P is the best option for slowing their degradation rate and reducing the gap between implant and native bone [26,27]. Besides Ca-P, Sr doped Ca-P coating on Mg better alloy performance in-vitro and in-vivo application. However, the stable degradation with the healing process still undergoes a challenged in the application of Mg alloy (Figure 2).
Figure 2:Surface modification of metallic implant with ceramic coating and polymeric layer for orthopedic application.
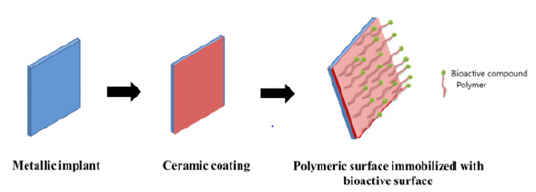
Current Challenges and Unmet Need for Biomaterials Derived Scaffold
Tissue engineering research utilizes ceramic, polymer, and metal to fabricate scaffold in bone regeneration application. For repair the hard tissue, mechanical strength and osteointegration of the scaffolds are required for long-term application. Ceramic scaffold mimics the bone ECM and provides support for cell growth, but their brittleness attributes make it difficult for the application. After sintering the ceramic scaffold, it should withstand sufficient mechanical strength with appropriate duration. Moreover, the porosity of ceramic scaffold is crucial for vascularization and cell movement. During ceramic-based scaffold design, vascularization, cell migration and nuclear transportation should be considered. Introduce polymers in the ceramic scaffolds is promising since it can overcome the limitation of ceramic scaffolds. In the polymeric hydrogel, the biocompatible crosslink is still challenging. Highly crosslinked polymers exhibit mechanical stability in repairing the hard tissue but delay the degradation rate. In addition, excessive crosslink agents showed cytotoxicity in in-vitro application. Further, their degradation rate effect on sustained release of drugs and immunogenicity. Metallic implants have multiple complications for their degradation and corrosion characteristic. Since Mg alloy overcome the postoperatives complications, their degradation induces toxicity in the defect site. Surface coating technology improves the performance of Mg alloy; however, still the osteoinductive coating layer is desirable to accelerate the wound healing process.
Conclusion
Biomaterials and their modification dramatically enhance the development of bio scaffold and advance the tissue engineering. Multidisciplinary research has undertaken to elicit better performance of biomaterials in scaffold fabrication. However, the right choice of biomaterials in respect to patient defect remain elusive, future research finds the appropriate options to overcome the existing problems.
References
- Bonucci E (2012) Bone mineralization. Front Biosci 17(1): 100-128.
- Arias CF, Herrero MA, Echeverri LF, Oleaga GE, Lopez JM (2018) Bone remodeling: A tissue-level process emerging from cell-level molecular algorithms. Plos One 3(9).
- Faruq O, Kim B, Padalhin AR, Lee GH, Lee BT (2017) A hybrid composite system of biphasic calcium phosphate granules loaded with hyaluronic acid-gelatin hydrogel for bone regeneration. J Biomater Appl 32(4): 433-445.
- Arzi B, Raine GD, Lee CA, Huey DJ, Borjesson DL, et al. (2015) Cartilage immunoprivilege depends on donor source and lesion location. Acta Biomater 23: 72-81.
- Nikolova MP, Chavali MS (2019) Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater 4: 271-292.
- Skorulska A, Piszko P, Rybak Z, Szymonowicz M, Dobrzynski M (2021) Review on polymer, ceramic and composite materials for CAD/CAM indirect restorations in dentistry-application, mechanical characteristics and comparison. Materials 14(7): 1596.
- Chia HN, Wu BM (2015) Recent advances in 3D printing of biomaterials. J Biol Eng 9: 4.
- Sayed S, Faruq O, Hossain M, Im SB, Kim YS, et al. (2019) Thermal cycling effect on osteogenic differentiation of MC3T3-E1 cells loaded on 3D-porous Biphasic Calcium Phosphate (BCP) scaffolds for early osteogenesis. Mater Sci Eng C Mater Biol Appl 105.
- Ebrahimi M, Botelho MG, Dorozhkin SV (2017) Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater Sci Eng C Mater Biol Appl 71: 1293-1312.
- Duta L, Dorcioman G, Grumezescu V (2021) A Review on biphasic calcium phosphate materials derived from fish discards. Nanomaterials 11(11): 2856.
- Cannio M, Bellucci D, Roether JA, Boccaccini DN, Cannillo V (2021) Bioactive glass applications: A literature review of human clinical trials. Materials 14(18): 5440.
- Hasan L, Kim B, Padalhin AR, Faruq O, Sultana T, et al. (2019) In vitro and in vivo evaluation of bioglass microspheres incorporated brushite cement for bone regeneration. Materials Science and Engineering: C 103.
- Zhang N, Kohn DH (2012) Using polymeric materials to control stem cell behavior for tissue regeneration. Birth Defects Res C Embryo Today 96(1): 63-81.
- Patel DK, Lim KT (2019) Biomimetic polymer-based engineered scaffolds for improved stem cell function. Materials 12(18): 2950.
- Bacakova L, Novotna K, Hadraba D, Musilkova J, Slepicka P, et al. (2022) Influence of biomimetically mineralized collagen scaffolds on bone cell proliferation and immune activation. Polymers 14(3): 602.
- Kozlowska J, Stachowiak N, Sionkowska A (2018) Collagen/gelatin/hydroxyethyl cellulose composites containing microspheres based on collagen and gelatin: Design and evaluation. Polymers (Basel) 10(4): 456.
- Kothale D, Verma U, Dewangan N, Jana P, Jain A, et al. (2020) Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr Drug Deliv 17(9): 755-775.
- Tomic SL, Nikodinovic-Runic J, Vukomanovic M, Babic MM, Vukovic JS (2021) Novel hydrogel scaffolds based on alginate, gelatin, 2-hydroxyethyl methacrylate, and hydroxyapatite. Polymers 13(6): 932.
- Vepari C, Kaplan DL (2007) Silk as a biomaterial. Prog Polym Sci 32(9): 991-1007.
- Lee HJ, Abueva CD, Padalhin AR, Lee BT (2020) Soya protein isolate-polyethylene oxide electrospun nanofiber membrane with bone marrow-derived mesenchymal stem cell for enhanced bone regeneration. J Biomater Appl 34(8): 1142-1149.
- Donnaloja F, Jacchetti E, Soncini M, Raimondi MT (2020) Natural and synthetic polymers for bone scaffolds optimization. Polymers 12(4): 905.
- Dwivedi R, Kumar S, Pandey R, Mahajan A, Nandana D, et al. (2020) Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J Oral Biol Craniofac Res 10(1): 381-388.
- Deng Y, Yang WZ, Shi D, Wu M, Xiong XL, et al. (2019) Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration. Nature 11(1): 1-13.
- Davies JE (2007) Bone bonding at natural and biomaterial surfaces. Biomaterials 28(34): 5058-5067.
- Yang X, Li M, Lin X, Tan L, Lan G, et al. (2013) Enhanced in vitro biocompatibility/bioactivity of biodegradable Mg–Zn–Zr alloy by micro-arc oxidation coating contained Mg2SiO4. Surface and Coatings Technology 233: 65-73.
- Xiao X, Zhu QS, Su YC (2013) In vitro degradation and biocompatibility of Ca-P coated magnesium alloy. Chem Res Chin Univ 29: 285-289.
- Ciobanu G, Carja G, Ciobanu O (2008) Structural characterization of hydroxyapatite layer coatings on titanium supports. Surface and Coatings Technology 202(11): 2467-2470.
© 2022 Omar Faruq. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






