- Submissions

Full Text
Orthoplastic Surgery & Orthopedic Care International Journal
CRISPR Technology in Africa: Challenges and Opportunities
Okpalaoka C1* and Onuselogu CC2
1Covenant University Center for Research Innovation and Discovery, Nigeria
2Department of Biological Sciences, Covenant University, Nigeria
*Corresponding author: Chijindu Okpalaoka, Department of Business Management, Ota Ogun State, Nigeria
Submission: April 01, 2022;Published: May 13, 2022
ISSN 2578-0069Volume2 Issue4
Opinion
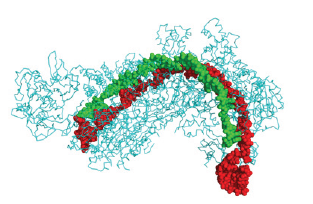
CRISPR is a family of DNA sequences present in the genes of bacterial species such as prokaryotic organisms [1]. These sequences are produced from fragments of DNA from microbes that infect the prokaryote. They are used to identify and repair genes from further infections and other viruses and bacteria. As a result, these sequences serve a critical role in prokaryotes’ antiviral (i.e., anti-phage) defensive system and provide a sort of acquired immunity [2]. CRISPR is present in around 50% of sequenced prokaryotes and almost 90% of sequenced eukaryotes [3]. Cas9 (or “CRISPR-associated protein 9) is an enzyme that employs CRISPR sequences to detect and cleave complementary DNA strands, as shown below (Figure 1). Cas9 enzymes in combination with CRISPR sequences constitute the core of the Crispr, which can be used to modify genes within organisms [4]. This editing approach offers a wide range of applications, including primary biology research, product development, and illness therapy [5]. The creation of the CRISPR-Cas9 genetic modification system by Emmanuelle Charpentier and Jennifer Doudna, was rewarded the 2020 Nobel Prize in Chemistry [6]. Due to the abundance of tropical diseases and pests, CRISPR is projected to significantly assist Africa’s public health, medicinal, and agricultural sectors. Malaria is the most prevalent tropical illness, accounting for over half a million deaths each year.
Figure 1:Cas9 (or “CRISPR-associated protein 9) is an enzyme that employs CRISPR sequences to detect and cleave complementary DNA strands.
Mosquitoes carry malaria and other diseases, yet efforts to control them have been unsuccessful for years due to the parasites’ biological complexity and the limitations of existing tactics. Recently, Clustered regularly interspaced short palindromic repeats genomes for malaria vectors and Anopheles gambiae were produced [7]. These strains could propagate antimalarial genes or decrease generations of wild populations because of their great nonmendelian inheritance. Though proofs-of-concept have not yet been tested on a broader scale in confinement or the field, their laboratory successes show a high promise for malaria elimination using CRISPR-based software [8]. On the other hand, African malaria is complicated, involving an: gambiae and other organisms. A holistic approach to malaria management would apply GDs (gene drives) for these other species, possibly with proper existing techniques. Additionally, GDs may be used to manage sicknesses, including Aedes aegypti, which spreads the dengue, chikungunya, and Zika viruses, for which effective vaccines or treatments are at this time unavailable. Similarly, inhabitants CRISPR-genetic drives would be appropriate in reducing dangerous infective pests in the region that require immediate but lack adequate, cost-effective, and feasible wide-area control, particularly the fall armyworm Spodoptera frugiperda, that wreaks havoc on major staplesii, and synonym invadens. Apart from gene drives and other prospective applications, Crispr is particularly well suited to healing major genetic illnesses in Africa, such as sickle-cell, which remained incurable until Ribeil and colleagues reported symptom remission in a patient by lentiviral gene therapy [9].
While this is a ground-breaking procedure, it is costly, timeconsuming, and only applicable postnatally, raising worries about after-treatment contaminations. Proofs-of-principle in rats and before grafting demonstrated the possibility of correcting sickle cell disease and other microbial mutations utilizing CRISPR or in combination with other methods [10], bolstering the argument that it could become the process of choice for effectual and costeffective pre- or postnatal DNA segment treatment for sickle cell in Africa. Clustered regularly interspaced short palindromic repeats may create vaccines against tropical diseases such as malaria, which are currently hampered by insufficient donor support and parasite complexity. However, irradiated trophozoites offer immunity against malaria vaccines based on their few mass production issues due to the low number of trophozoites ejected by mosquitoes. Another possibility, albeit unpleasant and undesirable, is bites from mosquitoes carrying irradiated sporozoites. These factors necessitate the deployment of alternative malaria vaccination techniques. Numerous genes contribute to the fight against Plasmodium infections in mosquitoes, and their silencing using RNA interference in anophelines modulates immunological pathways for or against the disease [11]. However, because RNA is transitory, RNA interference may not always wholly reduce RNA component. Crispr provides genetic DNA segment silencing and is preferable to RNAi for engineering parasites with abundant sporozoite creation for vaccinations. Plants and animals in Africa are at risk from a variety of cockroaches and illnesses. Breeding programs attempt to address these issues but have had limited success thus far. CRISPR could aid breeders in Africa in developing enhanced wildlife or plants that exhibit disease resistance or other desired characteristics. Recent genome editing in the tropical staple cassava [12] paves the way for increased efforts to achieve food security in Africa.
References
- Barrangou R, Doudna JA (2016) Applications of CRISPR technologies in research and beyond. Nat Biotechnol 34(9): 933-941.
- Makarova KS, Haft DH, Barrangou R (2011) Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9(6): 467-477.
- Lino CA, Harper JC, Carney JP, Timlin JA (2018) Delivering CRISPR: A review of the challenges and approaches. Drug Deliv 25(1): 1234-1257.
- Zhang F, Marraffini LA, Jiang W (2013) Multiplex genome engineering using CRISPR/Cas Systems. Science 339(6121): 816-819.
- Harrison MM, Jenkins B V, O’Connor-Giles KM, Wildonger J (2014) A CRISPR view of development. Genes Dev 28(17): 1859-1872.
- Fernholm A, Barnes C (2020) Genetic Scissors : A tool for rewriting the code of life. The Nobel Prize in Chemistry 2020.
- Gantz VM, Jasinskiene N, Tatarenkova O (2015) Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A 112(49): E6736-E6743.
- Carballar-Lejarazú R, James AA (2017) Population modification of Anopheline species to control malaria transmission. Pathog Glob Health 111(8): 424-435.
- Ribeil J-A, Hacein-Bey-Abina S, Payen E (2017) Gene therapy in a patient with sickle cell disease. N Engl J Med 376(9): 848-855.
- Ma H, Marti-Gutierrez N, Park SW (2017) Correction of a pathogenic gene mutation in human embryos. Nature 548(7668): 413-419.
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G (2010) Mosquito immune defenses against plasmodium infection. Dev Comp Immunol 34(4): 387-395.
- Odipio J, Alicai T, Ingelbrecht I, Nusinow DA, Bart R, et al. (2017) Efficient CRISPR/cas9 genome editing of phytoene desaturase in cassava. Front Plant Sci 8: 1-11.
© 2022 Okpalaoka C. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






