- Submissions

Full Text
Orthoplastic Surgery & Orthopedic Care International Journal
Wound Bed Preparation: Standards of Care and Predictability of the Healing Time of Chronic Wounds
Claudio Ligresti, Fabio Sasso, Emiliano Jahaj and Erind Ruka*
Department of Reconstructive and Aesthetic Plastic Surgery, Maria Pia Hospital, Italy
*Corresponding author: Erind Ruka, Department of Reconstructive and Aesthetic Plastic Surgery, Maria Pia Hospital, Turin, Italy
Submission: February 15, 2018;Published: September 07, 2018
ISSN 2578-0069Volume2 Issue1
Abstract
We intend to highlight the need to establish a hypothetical time of healing for chronic wounds. Such innovative aspect within the TIME acronym [tissue, infection/inflammation, moisture balance and edge of wound] is based on a more correct analysis of the general and local symptoms that the patient presents, classifying them in different ways according to their characteristics. The analysis of medical history, pathogenesis and clinical leads to a total score that can direct the clinician to choose principals important and aggressive that can be used according to basic concepts.
Background
To predict the healing time for each patient is not always a simple task. The healing process is the result of a complex interaction between factors related to injury, the treatment used, skills and knowledge of the nursing staff. The best way to shorten the healing time is an accurate staging of the lesion [therefore a proper understanding of the gravity of the wound] and then the decision of the most appropriate therapy. In the last twenty years there have been great strides in the understanding of the biological mechanisms involved in repairing wounds, so now more than ever we have the means necessary to assume a time of complete healing as accurate as possible [1].
Since 2007, Ligresti et al. [2], with the introduction of the TIME - H, relate the healing time with the type of therapy used: it focuses on how the therapy is used with the same type of lesion to make a difference in healing time. Starting in 2008 with the EWMA position document [3] a holistic approach to wound healing is considered but also the complexity of the wounds, the healing process, psychosocial factors involved in healing and economic burden of chronic wounds are considered. Our paper work tends to make available to the medical staff a valid model showing the most correct treatment for a certain type of injury that can allow us to predict the healing time.
Material and Methods
We performed a study on a group of 40 patients, enlisting 28 females, 12 males aged between 30-87 years. The injuries were wounds and skin ulcers of similar size [50-100cm2], pressure ulcers, venous leg ulcers, diabetic foot, PDS posttraumatic. All patients were treated with one of the principals randomly choosing among those listed with intervals recommended by the type of wound. The study duration was 6 months.
In our proposal we considered a whole TIME, pain conditions, environmental conditions and lifestyle to get a severity index [IG], which allows us to have a prediction of healing time. The severity rate also allows us to adopt different therapeutic strategies based on the severity of various injuries Starting from TIME we built a scale of values that we added from a maximum score of 16 [2-6]. Then, we considered the volume [volume “W” single ulcer or the sum of multiple ulcers]. As shown by Kramer et al. [7] the size and depth of pressure ulcers are good predictors of healing: as lower as the ulcer grade is greater will be the chance of recovery.
We then moved on to the study of pain considering parameter with score 1 as no pain, mild pain score with value 2 [stimulated during the dressing value and 3 to intense pain [without stimulation] [8]. Finally, we look at general conditions and environmental factors [9-24]. At this point in our algorithm we add the Time and the FAC [factors, anamnestic, social and clinical] and multiply them for pain and volume: the value obtained is our gravity index [IG].
TIME [1-16] + FAC [1-18] x PAIN [1-3] x VOLUME [2-32] = IG
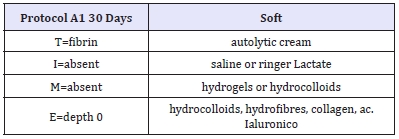
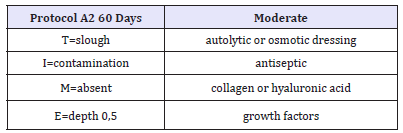
The healing time [TH], as already stated, also depends on the therapeutic strategy undertaken: the numerical value is in direct relation with the therapy. The TH is=to IG and will not change if the therapeutic choice [soft] resides on the left side of the table; is < 30% of IG if therapy undertaken [medium aggressive] is in the middle of the table; is < 60% of IG if therapy choice [aggressive] is on the right side of the table. We assume that we are faced with an ulcer from healing [healing time >60 days]. We can change the situation by customizing the type of therapy on the individual case. In this way we can modify the healing time [4].
Take for example a lesion volume between 50 and 100cm3. We can have a lesion T0, I0, M0, E0, thus devoid of necrotic tissue, bacterial contamination, exudate and with re-epithelialisation rate >75%, or a lesion T4, I4, M4 and E4 with 100% of necrotic tissue infections, exudate and the absence of spontaneous reepithelialization: with the same situation, in any case, the choice of therapy will change the history of the lesion and the timing of healing. For example, the T, we can see how, by choosing a soft treatment [such as autolysis, we have a significant improvement of the lesion as low as 25 days [25-27]. This time can decrease, even drastically, by level up in the type of strategy used: the time passes to 20 days with osmosis, 15 days with the larvae, until a day using hydrotherapy, ultrasound or surgery. Regarding the I: we passed from an improvement of 55 days with saline [strategy blander], to 21 days of antiseptics/ dressings with silver/NPWT [negative pressure wound therapy] up to 7 days after the surgery/ antiseptics/antibiotics/NPWT. The M represents an increase in 55 days with hydrogel/hydrocolloid, hydrofibre 28 days with up to 10 days of the surgery. Finally, the E: re-epithelialization from 100 days with advanced medications, VAC 45 days with up to 10 days with autologous graft [28-56].
Time of Healing
The numerical value is in direct relation to the selected therapy.
It is=to that of IG patient and its value will not change if the therapy choice [soft] is in the left side of the table therapeutic
It is < 30% of IG patient if treatment choice [medium-aggressive] is in the centre of the table therapeutic
It is < 60% of IG patient if treatment choice [aggressive] is on the right side of the table.
Results and Discussion
The test results have shown that the margin of error in predicting the healing time was < 10% of the 40 patients analysed in detail (Table 1):
Table 1:Soft: not aggressive treatment.
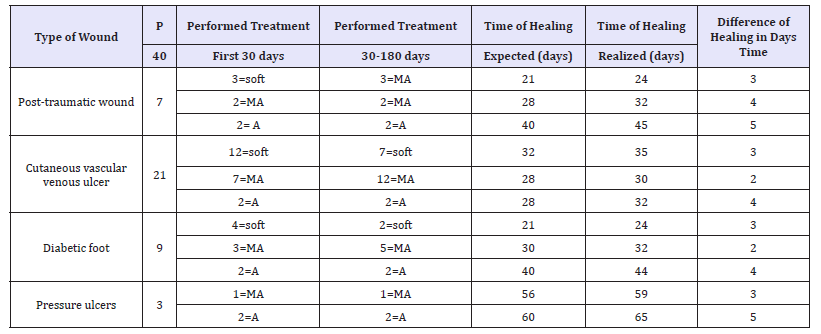
MA: Medium-Aggressive Treatment; A: Aggressive Treatment
Report of the results obtained with the averages of the healing times on a number of 22 patients, obtaining a slightly higher percentage of error of 10% in the prediction of healing time.
Our protocol
GI is < 23=and ASA 1-5 soft therapy
GI is 24-50 and ASA < 4 with infection between 1-2=mediumaggressive therapy
GI is >50 and ASA < 4 with infection between 3-4=aggressive therapy
GI is >50 and ASA is 4-5 with infection between 1-2=soft therapy
GI is >50 and ASA is 4-5 with infection between 3-4=mediumaggressive therapy
Discussion
Nowadays, various systems today try to give a prediction of healing time, taking into account various parameters. Troxler et al. [4] studied the importance of periodic evaluations of the wound, accompanied by measurements of its surface, for the identification of potentially hard-to-heal wounds. The early detection of a reduction in the size of the wound is set by measuring the progress of the margin [epithelial advancement]. Phillips et al. [57] considering the percentage reduction in venous ulcer area found that in about 77% of cases, healing outcomes could be predicted based on a wound size reduction of more than 44% at three weeks. Margolis et al. [58,59] were able to show that for venous leg ulcers a simple rating system score based on size and duration can give a good indication of the likely outcome at 24 weeks. Falanga et al. [60] incorporated measurement of epithelial advancement into a scoring system on the healing of venous leg ulcers. This system [wound bed score] also examines other characteristics including the extent of skin dermatitis around the wound, the presence of eschar, callus and/or fibrosis around a wound, pink or red wound bed, exudate and the volume of the edema (Table 2).
Table 2:
The complexity of the wound is likely to exert a significant influence on the progression of the healing process, and the factors that combine to determine it can be classified into four main groups: patient factors, factors related to the wound, knowledge of the HCP, factors and resources related to the treatment. In a study by Margolis et al. on a group of patients with venous ulcers, it has emerged a correlation between some specific characteristics of the wound and the healing process: wound duration, size, depth of the wound]. Ulcer size [>2cm2], the duration [>two months] and depth [penetration through exposed tendon, ligament, bone or joint] were the three most important factors for predicting the outcome. Patients with all three factors had only a 22% chance of healing by 20 weeks (Table 3).
Table 3:
For the physiological nature of the healing process, it is inevitable that large wounds will require more time to heal than smaller wounds. In addition, the longer a wound remains open, the greater the risk of complications, such as infections, is present. Therefore, a treatment that reduces the size of the wound and the infection risk is able to offer potential benefits. The presence of necrotic tissue in a wound has been for a long time considered an obstacle to the evaluation of the lesion, as well as a potential predictive factor of delayed healing and a possible outbreak of infection (Table 4).
Table 4:
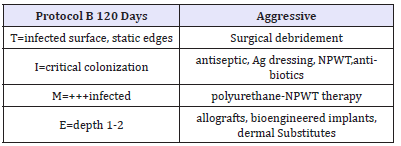
Table 5:Soft: not aggressive treatment.
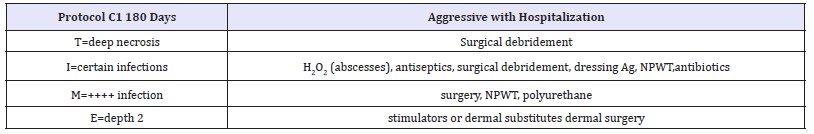
In chronic wounds, there is a tendency for the inflammatory response [which is an important element of the initial response to the lesion]. This results in increased production of pro inflammatory cytokines, reactive oxygen species and proteolytic enzymes [such as certain MMPs, elastase and plasmin]. This activity is combined with a minor issue, for example, inhibitors TIMP (Table 5), and is further enhanced by alterations of pH at the level of the wound bed. Excessive activity of these enzymes causes not only deleterious extracellular matrix destruction, but also inactivation of growth factors. There is a correlation between the state of chronic inflammation of a wound, the high levels of protease exudate and the slowdown in the process of tissue repair. The control of inflammation and the concentration of MMPs [metalloproteases are essential, as the protease, not only degrade the fabric, but lead to malnutrition of the growth factors [61-69].
Table 6:Soft: not aggressive treatment.
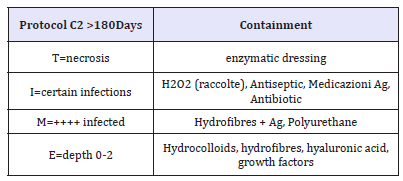
Gjødsbøl et al. [70] found a significant link between diversity and the density of the bacterial species detected on the diagnostic buffer and the time required for wound healing. Also, the presence in a wound of specific bacterial species has been put in relation with the outcome of healing. For example, the presence of Pseudomonas aeruginosa in venous leg ulcers can delay healing. According Mogford et al. [71], an ischemic wound is probably the most common cause of non-healing. Because of a poor perfusion, metabolic gas exchange at the level of tissues become inefficacious. It has been shown that the healing of a wound following surgery is compromised by dehydration and by a low body temperature of the patient, factors that are associated with reduced perfusion tissue and poor oxygenation (Table 6).
Physical factors such as diabetes mellitus, obesity, malnutrition, advanced age [over 60], decreased perfusion, peripheral vascular disease, cancers, organ failure, sepsis, and even restrictions mobility, can affect the healing process. Marston et al. [72] have found that improved glycemic control has a positive influence on the outcome in diabetic foot wounds, particularly when dermal substitutes are used. Terms of immunodeficiency, use of immunosuppressive drugs [corticosteroids, azathioprine or methotrexate] [73-75] or the presence of diseases [such as diabetes mellitus] [76-79] known to affect the immuno-inflammatory response, are all circumstances that may influence negatively healing and increase the risk of wound sepsis.
It was also found that psychosocial factors [80-82], such as social isolation, gender, smoking, the economic conditions and the experience of pain could influence wound healing. Stress and depression have been linked to changes in immune function and may therefore adversely influence a wide range of physiological processes, including wound healing. In a human experimental model, it was found that stress and depression had a possible role in the modulation of matrix metalloproteinases [MMPs] and expression of tissue inhibitors of metalloproteinases [TIMP]. According to some studies also the ability to cope with stress is a factor that can influence healing times. Salaman et al. [83] studied a group of 45 hospital patients with venous ulcers, 16 [36%] of who do not make satisfactory progress. Only half of these 16 patients said they have received any explanation about the cause of the ulcer and the treatment method used. This study raises important questions about the impact on wound healing of the patient’s beliefs and their confidence in treatment. Blunting is the case of patients who are indifferent to the processing and not very interested in the progress of the wound towards the healing.
Although the feeling of helplessness experienced by some patients, many of them make every effort to ensure that the care they receive meets their needs. Some patients become experts in their own condition, often using the Internet to gather information on it. When a wound is located on a pressure-bearing surface or a mobile area such as around a joint, the choice of the material of the dressing and the method of attachment is of in extreme importance. However, Chipchase et al. [84] observed that, while the overall healing rates of foot ulcers were similar, lesions located in the heel tended to heal more slowly. The authors concluded that the outcome was generally favorable, with 65.6% of heel ulcers healed in a median time of 200 days.
Harding [85] has the potential effect of fibroblast senescence on chronic wound healing. There was a correlation between the ratio of senescent fibroblasts/non-senescent fibroblasts and healing outcomes: an accumulation of more than 15% senescent fibroblasts is considered the threshold beyond which wounds will have trouble healing. Moreover, the response to treatment can be an indicator of tissue viability and healing potential. For example, it was suggested that a reduction in wound area of around 15% within one to two weeks of topical negative pressure therapy is a positive indicator of the likely evolution of the wound, and that this observation can be the decision to continued therapy.
As seen above, many attempts and proposals tend to quantify the time required for wound healing. On the basis of previous attempts, our aim is to propose an algorithm not only to accurate the quantification of healing time, but also a precise protocol of treatment to be associated with any situation and any type of wound. It is therefore clear, as the challenge of healing of a skin lesion, cannot be achieved by a single specialist. It’s fundamental the collaboration between various specialists. It’s obvious that the planning therapy must be organised by a team leader to coordinate the rest of the medical staff [various specialists including plastic surgeon, vascular surgeon, the internist, the nutritionist, the endocrinologist, the dermatologist, etc.] and not by hospital nurses or domiciles medical figures. Only with careful collaboration and clearly careful training you can pursue the goal in the shortest time possible and with a greatest patient comfort.
This protocol sets itself as a general guideline of the ulcer treatment for all types of professionals involved in the management of the patient but is particularly useful for those who approach the world of vulnology [wound treatment], but who do not yet have a detailed knowledge about. It appears a useful tool, since for the first time, it offers a mathematical model in which a score is calculated and according to the same applies a type of therapy.
Conclusion
The algorithm that we propose is a useful tool for staging the severity of injuries and provides a simple means to adjust therapy. It describes how the therapy used should change significantly according to the type and severity of the wound that we are facing and how this in turn can affect significantly the healing time estimated. It’s clear that, not always, the mathematical calculations are an exact prediction, but allow however to have a prediction of the initial situation. There are margins of error, especially when it takes over factors that complicate the wound management. It is highly appreciated that, to predict the outcome of recovery in individual patients, it will be necessary to use not a single marker, but the data resulting from a combination of several markers, as we proposed. Many authors have stressed the importance of training the nursing staff, designed to provide the knowledge and skills necessary to establish appropriate treatment and process protocols and forms relating to wound care [56].
However, we can conclude by saying that we do not presume to have created a perfect model for the treatment of skin lesions, we only want to provide a useful tool to the caregiver based on current knowledge and on current therapeutic strategies. We are still waiting for new knowledge that can lead us to an even higher level in the treatment of this complex disease.
References
- Chase SK, Melloni M, Savage A (1997) A forever healing: the lived experience of venous ulcer disease. J Vasc Nurs 15(2): 73-78.
- Ligresti C, Bo F (2007) Wound bed preparation of difficult wound: an evolution of principles of TIME. Int Wound J 4(1): 21-29.
- P Vowden J, Apelqvist C, Moffatt (2008) Hard to heal wounds: a holistic approach. EWMA.
- Bailey MA, Mc Pherson SJ, Troxler MA, Peach AH, Patel JV, et al. (2011) Ischemic skin ulceration complicating glue embolization of type II endoleak after endovascular aneurysm repair. J Vasc Interv Radiol 22(2): 163-167.
- Gottrup F (2002) Oxygen, wound healing and the development of infection. Present status. Eur J Surg 168(5): 260-263.
- Bux M, Malhi JS (1996) Assessing the use of dressings in practice. J Wound Care 5(7): 305-308.
- Kramer JD, Kearney M (2000) Patient, wound, and treatment characteristics associated with healing in pressure ulcers. Adv Skin Wound Care 13(1): 17-24.
- Franks PJ, Moffatt CJ (2006) Do clinical and social factors predict quality of life in leg ulceration?. Int J Low Extrem Wounds 5(4): 236-243.
- Moor AN, Tummel E, Prather JL, Jung M, Lopez JJ, et al. (2014) Consequences of age on ischemic wound healing in rats: altered antioxidant activity and delayed wound closure. Age (Dordr) 36(2): 733- 748.
- Margolis DJ, Berlin JA, Strom BL (1999) Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 135(8): 920-926.
- McGinnis E, Greenwood DC, Nelson EA, Nixon J (2014) A prospective cohort study of prognostic factors for the healing of heel pressure ulcers. Age Ageing 43(2): 267-271.
- Little MO (2013) Nutrition and skin ulcers. Curr Opin Clin Nutr Metab Care 16(1): 39-49.
- Leymarie F, Richard JL, Malgrange D (2005) Factors associated with diabetic patients at high risk for foot ulceration. Diabetes Meta; 31(6): 603-605.
- Kiguchi MM, Hager ES, Winger DG, Hirsch SA, Chaer RA, et al. (2014) Factors that influence perforator thrombosis and predict healing with perforator sclerotherapy for venous ulceration without axial reflux. J Vasc Surg 59(5): 1368-1376.
- Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3): 219-229.
- Costa A, Cunha M (2013) [Prevalence of pressure ulcers in person victim of trauma: predisposing factors]. Servir 58(1-2): 60-77.
- Abbade LP, Lastória S, Rollo Hde A (2011) Venous ulcer: clinical characteristics and risk factors. Int J Dermatol 50(4): 405-411.
- Ahmad W, Khan IA, Ghaffar S, Al-Swailmi FK, Khan I (2013) Risk factors for diabetic foot ulcer. J Ayub Med Coll Abbottabad 25(1-2): 16-18.
- Baba M, Davis WA, Davis TM (2014) A longitudinal study of foot ulceration and its risk factors in community-based patients with type 2 diabetes: The Fremantle Diabetes Study. Diabetes Res Clin Pract 106(1): 42-49.
- Astudillo B, Cruz M, del Prado L, Domenack R, Nasi E, et al. (2013) Profile of patients admitted with infected skin ulcers at Bella Vista Hospital Mayagüez. Bol Asoc Med P R 105(3): 29-35.
- Haji Zaine N, Burns J, Vicaretti M, Fletcher JP, Begg L, et al. (2014) Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res 7(1): 39.
- Huliev D (2013) [Obstacles in wound healing]. Acta Med Croatica 67(Suppl 1): 5-10.
- Raju D, Su X, Patrician PA, Loan LA, McCarthy MS (2014) Exploring factors associated with pressure ulcers: A data mining approach. Int J Nurs Stud 52(1): 102-111.
- Peghetti A, Mantovani M, Canova G, Ferri L (2012) Le medicazioni avanzate per il trattamento delle ferite acute e croniche. Dalle evidenze della letteratura alla pratica quotidiana. Commissione Regionale Dispositivi Medici Reg Emilia Romagna pp. 1-126.
- AISLeC (1995) Profilassi delle lesioni da decubito e cambio posturale: ricerca multicentrica. AISLeC.
- Bateman S (2014) Principles of preventative foot care. Br J Community Nurs Suppl: S30, S32-4, S36-8.
- Gantwerker EA, Hom DB (2011) Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am 19(3): 441-453.
- (2011) Registered Nurses Association of Ontario Risk Assessment and Management of Pressure Ulcers.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR (2010) A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 39(6): 1065-1076.
- Dumville JC, Deshpande S, O’Meara S, Speak K (2011) Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev (9): CD009111.
- Steenfos HH, Agren MS (1998) A fibre-free alginate dressing in the treatment of split thickness skin graft donor sites. J Eur Acad Dermatol Venereol 11(3): 252-256
- Terrill PJ, Goh RC, Bailey MJ (2007) Split- thickness skin graft donor sites: a comparative study of two absorbent dressings. J Wound Care 16(10): 433-438.
- Belmin J, Meaume S, Rabus MT, Bohbot S (2002) Sequential treatment with calcium alginate dressings and hydrocolloid dressings accelerates pressure ulcer healing in older subjects: a multicenter randomized trial of sequential versus nonsequential treatment with hydrocolloid dressings alone. J Am Geriatr Soc 50(2): 269-274.
- Meaume S, Ourabah Z, Cartier H, Granel- Brocard F, Combemale P, et al. (2005) Evaluation of a lipidocolloid wound dressing in the local management of leg ulcers. J Wound Care 14(7): 329-334.
- Palfreyman S, Nelson EA, Michaels JA (2007) Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ 335(7613): 244.
- Gethin G, Cowman S (2009) Manuka honey vs. Hydrogel-a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. J Clin Nurs 18(3): 466-474.
- Valenzuela AR, Perucho NS (2008) The effectiveness of a 0.1% polyhexanide gel. Rev Enferm 31(4): 7-12.
- Edwards J, Stapley S (2010) Debridement of diabetic foot ulcers. Cochrane Database Syst Rev (1): CD003556.
- Abbruzzese L, Rizzo L, Fanelli G, Tedeschi A, Scatena A, et al. (2009) Effectiveness and safety of a novel gel dressing in the management of neuropathic leg ulcers in diabetic patients: a prospective double- blind randomized trial. Int J Low Extrem Wounds 8(3): 134-140.
- Hien NT, Prawer SE, Katz HI (1988) Facilitated wound healing using transparent film dressing following Mohs micrographic surgery. Arch Dermatol 124(6): 903-906.
- Lo SF, Hayter M, Chang CJ, Hu WY, Lee LL (2008) A systematic review of silver-releasing dressings in the management of infected chronic wounds. J Clin Nurs 17(15): 1973- 1985.
- Andriessen A, Polignano R, Abel M (2009) Monitoring the microcirculation to evaluate dressing performance in patients with venous leg ulcers. J Wound Care 18(4): 145-150.
- Li-Tsang CW, Lau JC, Choi J, Chan CC, Jianan L (2006) A prospective randomized clinical trial to investigate the effect of silicone gel sheeting (Cica-Care) on post- traumatic hypertrophic scar among the Chinese population. Burns 32(6): 678-683.
- Harte D, Gordon J, Shaw M, Stinson M, Porter-Armstrong A (2009) The use of pressure and silicone in hypertrophic scar management in burns patients: a pilot randomized controlled trial. J Burn Care Res 30(4): 632- 642.
- Trial C, Darbas H, Lavigne JP, Sotto A, Simoneau G, et al. (2010) Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. J Wound Care 19(1): 20-26.
- Bishop JB, Phillips LG, Mustoe TA, VanderZee AJ, Wiersema L, et al. (1992) A prospective randomized evaluator-blinded trial of two potential wound healing agents for the treatment of venous stasis ulcers. J Vasc Surg 16(2): 251-257
- Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, et al. (2009) Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 13(54): 1-86.
- Wasiak J, Cleland H, Campbell F (2008) Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev (3): CD002106.
- Preece J (2004) Development of a wound-management formulary for use in clinical practice. Prof Nurse 20(3): 27-29.
- Gravante G, Delogu D, Giordan N, Morano G, Montone A, et al. (2007) The use of Hyalomatrix PA in the treatment of deep partial-thickness burns. J Burn Care Res 28(2): 269-274.
- Vowden P, Romanelli M, Price P (2007) Effect of amelogenin extracellularmatrix protein and compressionon hard-to- heal venous leg ulcers. Journal of Wound Care 16(5): 189-195.
- Piaggesi A, Goretti C, Mazzurco S, Tascini C, Leonildi A, et al. (2010) A randomized controlled trial to examine the efficacy and safety of a new super-oxidized solution for the management of wide postsurgical lesions of the diabetic foot. Int J Low Extrem Wounds 9(1): 10-15.
- Maume S, Van De Looverbosch D, Heyman H, Romanelli M, Ciangherotti A, et al. (2003) A study to compare a new self-adherent soft silicone dressing with a self-adherent polymer dressing in stage II pressure ulcers. Ostomy Wound Manage 49(9): 44-51.
- Piatkowski A, Drummer N, Andriessen A, Ulrich D, Pallua N (2011) Randomized controlled single center study comparing a polyhexanide containing bio-cellulose dressing with silver sulfadiazine cream in partial-thickness dermal burns. Burns 37(5): 800-804.
- König M, Vanscheidt W, Augustin M, Kapp H (2005) Enzymatic versus autolytic debridement of chronic leg ulcers: a prospective randomised trial. J Wound Care 14(7): 320-323.
- Maggio G, Armenio A, Ruccia F, Giglietto D, Pascone M, et al. (2011) A new protocol for the treatment of the chronic venous ulcers of the lower limb. Clin Exp Med 12(1): 55-60.
- Harding KG, Moore K, Phillips TJ (2005) Wound chronicity and fibroblast senescence-implications for treatment. Int Wound J 2(4): 364-368.
- Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ (2009) Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 17(3): 318-325.
- Panuncialman J, Falanga V (2009) The Science of Wound Bed Preparation. Surg Clin North Am 89(3): 611-626.
- Margolis DJ, Berlin JA, Strom BL (1999) Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 135(8): 920-926.
- Xue M, Le NT, Jackson CJ (2006) Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 10(1): 143-155.
- Ravanti L, Kahari VM (2000) Matrix metalloproteinases in wound repair (review). Int J Mol Med 6(4): 391-407.
- Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinases A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem 271(29): 17119-17123.
- Di Colandrea T, Wang L, Wille J, D’Armiento J, Chada KK (1998) Epidermal expression of collagenase delays wound-healing in transgenic mice. J Invest Dermatol 111(6): 1029-1033.
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, et al. (1993) Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 92(6): 2858-2866.
- Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, et al. (1996) Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 135(1): 52-59.
- Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR (1998) Dynamics of the matrix metallo- proteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen 6(2): 127-134.
- Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, et al. (2002) Expression of matrix-metalloprotei- nases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 45(7): 1011-1016.
- Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, et al. (2002) Ratios of activated matrix metalloprotei- nase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 10(1): 26-37.
- Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, et al. (2006) Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3(3): 225-232.
- Mogford JE, Mustoe TA (2001) Experimental models of wound healing. In: Falanga V (Ed.), Cutaneous Wound Healing. Martin Dunitz Ltd, London, UK.
- Marston WA, Dermagraft Diabetic Foot Ulcer Study Group (2006) Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia. Ostomy Wound Manage 52(3): 26- 28,30,32.
- Wang AS, Armstrong EJ, Armstrong AW (2013) Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg 206(3): 410-417.
- Van Houtum WH, Lavery LA (1996) Outcomes associated with diabetesrelated amputations in the Netherlands and in the state of California, USA. J Intern Med 240(4): 227-231.
- Tiganescu A, Hupe M, Uchida Y, Mauro T, Elias PM, et al. (2014) Increased glucocorticoid activation during mouse skin wound healing. J Endocrinol 221(1): 51-61.
- Sørensen LT (2012) Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg 255(6): 1069-1079.
- Sievert H, Evers T, Matevossian E, Hoenemann C, Hoffmann S, et al. (2013) The influence of lifestyle (smoking and body mass index) on wound healing and long-term recurrence rate in 534 primary pilonidal sinus patients. Int J Colorectal Dis 28(11): 1555-1562.
- Nassaji M, Askari Z, Ghorbani R (2014) Cigarette smoking and risk of pressure ulcer in adult intensive care unit patients. Int J Nurs Pract 20(4): 418-423.
- Moura Neto A, Zantut-Wittmann DE, Fernandes TD, Nery M, Parisi MC (2013) Risk factors for ulceration and amputation in diabetic foot: study in a cohort of 496 patients. Endocrine 44(1): 119-124.
- Mathus-Vliegen EM (2004) Old age, malnutrition, and pressure sores: an ill-fated alliance. J Gerontol A Biol Sci Med Sci 59(4): 355-360.
- Franks PJ, Bosanquet N, Connolly M, Oldroyd MI, Moffat CJ, et al. (1995) Venous ulcer healing: effect of socioeconomic factors in London. J Epidemiol Community Health 49(4): 385-388.
- Abbade LP, Lastoria S, de Almeida Rollo H, Stolf HO (2005) A sociodemographic, clinical study of patients with venous ulcer. Int J Dermatol 44(12): 989-992.
- Salaman RA, Harding KG (1995) The aetiology and healing rates of chronic leg ulcers. J Wound Care 4(7): 320-323.
- Chipchase SY, Treece KA, Pound N, Game FL, Jeffcoate WJ (2005) Heel ulcers don’t heal in diabetes. Or do they?. Diabet Med 22(9): 1258-1262.
- Harding K, Cutting K, Price P (2000) The cost-effectiveness of wound management protocols of care. Br J Nurs. Oct;9(19 Suppl): S6, S8, S10 passim.
© 2018 Erind Ruka. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






