- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Right to Left Angina Yasser’s Syndrome (Swinging Yasser’s Central Heart Syndrome) or Dancing Yasser’s Heart Syndrome-A New Cardiovascular Discovery and Differentiation
Yasser Mohammed Hassanain Elsayed*
Critical Care Unit, Kafr El-Bateekh Central Hospital, Damietta, Egyptian Ministry of Health (MOH), Egypt
*Corresponding author: Yasser Mohammed HE, Critical Care Unit, Kafr El-Bateekh Central Hospital, Damietta, Egyptian Ministry of Health (MOH), Egypt
Submission: January 25, 2024;Published: March 13, 2024

ISSN 2578-0204Volume4 Issue3
Abstract
Rationale: Mesocardia is the heart in the middle compartment of the chest. The human heart is normally
located within the thoracic cavity, medially between the lungs in the mediastinum. Marfan syndrome is
an autosomal dominant disorder and multi-systemic genetic disorder that affects the connective tissue.
Dextrocardia is a rare congenital condition in which the apex of the heart is located on the right side of
the body rather than towards the left. Heterotaxy syndromes refer to abnormal left/right distribution
of thoracic and abdominal organs that is neither situs solitus nor situs inversus. They are commonly
associated with congenital heart disease (CHD) and visceral malformations. There is either left or right
isomerism also present in Heterotaxy syndromes.
Patient concerns: A 17-year-old adolescent single-student male patient was presented to the intensive
care unit (ICU) with angina and alternation of the chest pain referral to both arms.
Diagnosis: Right to left angina Yasser’s syndrome (Swinging Yasser’s central heart syndrome) or Dancing
Yasser’s heart syndrome in an adolescent male patient.
Interventions: Electrocardiography and echocardiography.
Outcomes: Spontaneous dramatic clinical, and electrocardiographic improvement with no medications
had happened.
Lessons: Right to left angina Yasser’s syndrome (Swinging Yasser’s central heart syndrome) or Dancing
Yasser’s heart syndrome is a new and innovative cardiovascular syndrome. Due to some similarities,
dextrocardia, Marfan syndrome, and Heterotaxy syndrome are implicated in Differentiation. Dancing
hyperactivity, traction, and twisting theories are interpretative suggested theories for this new syndrome.
Keywords:Mesocardia; Marfan syndrome; Dextrocardia; Heterotaxy syndromes; Axis shifting; Right to left angina Yasser’s syndrome; Swinging Yasser’s central heart syndrome; Dancing Yasser’s heart syndrome
Abbreviations:CBC: Complete Blood Count; CHD: Congenital Heart Disease; CXR: Chest Radiography; ECG: Electrocardiography; IHD: Ischemic Heart Disease; ICU: Intensive Care Unit; MFS: Marfan Syndrome; RAD: Right Axis Deviation; SGOT: Serum Glutamic-Oxaloacetic Transaminase; SGPT: Serum Glutamic- Pyruvic Transaminase; VR: Ventricular Rate
Introduction
In humans, the heart is normally located between the lungs and in the middle compartment of the chest [1]. The cardiac position in the thorax may be described as levocardia which is the left-sided heart dextrocardia which is the right-sided heart, and mesocardia which is the midline heart [2]. Mesocardia is extremely rare and accounts for only 0.2% of congenital anomalies [3]. Dextrocardia is a rare congenital condition in which the apex of the heart is located on the right side of the body, rather than the more typical placement towards the left [4]. Dextrocardia refers to a heart positioned on the right side of the chest. Three types of dextrocardia were described: 1. An isolated dextrocardia or dextrocardia of embryonic arrest in which the heart is in right in the thorax than is normal. It is commonly associated with pulmonary hypoplasia. 2. Dextrocardia situs solitus in which the heart is on the right side of the chest with viscera that are in the normal position and the stomach on the left side. 3. And dextrocardia situs inversus in which the positions of the abdominal organs and viscera are reversed. Cardiac dextroposition can result from hypoplasia of the right lung or a left-diaphragmatic hernia or eventration. Chest radiography (CXR) confirmed that her heart was in the right chest and that the cardiac apex pointed to the right. The aortic arch was on her left; mediastinal contours were normal; her lungs were clear; and the gastric bubble was on her left side [5].
To recognize the ECG changes associated with dextrocardia, it is important to have a clear understanding of the electrical axis. Global negativity in lead I (a negative P-wave, QRS complex, and T-wave), positively deflected QRS-complex in aVR, negative P-wave in lead II, reverse R-wave progression in precordial leads, and right axis deviation (RAD) [6]. Marfan syndrome (MFS) is a multi-systemic genetic disorder that affects the connective tissue (CT) [7,8]. MFS is caused by a mutation in FBN1, one of the genes that make fibrillin, which results in abnormal connective tissue. It is an autosomal dominant disorder [9]. They also typically have exceptionally flexible joints and abnormally curved spines. Patients of MFS may be tall and thin and have long arms, legs, fingers, and toes, as well as flexible joints. The most serious complications of MFS include the heart and aorta, with an increased risk of mitral valve prolapse (MVP) and aortic aneurysm. Some symptoms of MFS may be visible to others: a chest that sinks in or sticks out, a long head with deep-set eyes, a tall, thin body, flat feet, flexible joints, long arms, legs, fingers, and toes. Eye problems including blurred vision or trouble seeing things that are far away, are often the first sign of MFS. These can be caused by the lens in one or both eyes moving out of place. Palpitations are common. Pain can occur in variant parts of the body, especially, in the lower back scoliosis. Stretch marks may appear on the skin of the lower back, buttocks, shoulders, breasts, thighs, and stomach area. Teeth that become too crowded or weak are more likely to break or have cavities [9]. MFS commonly involves cardiovascular, ocular, and musculoskeletal systems with a wide range of manifestations, such as ascending aorta aneurysms and dissection, mitral valve prolapses, ectopia lentis, and long bone overgrowth [10]. The following conditions may result from MFS, but may also occur in people without any known underlying disorder; aortic aneurysm or dilation, arachnodactyly, GERD, bicuspid aortic valve, cysts, cystic medial necrosis, degenerative disc disease, deviated septum, dural ectasia, early cataracts, early glaucoma, early osteoarthritis, ectopia lentis, emphysema, iris coloboma, above-average height, palpitations, hernias, high-arched palate, hypermobility of the joints, kyphosis, leaky heart valve malocclusion, micrognathia, mitral valve prolapse, myopia, obstructive lung disease, osteopenia, pectus carinatum or excavatum, pes planus, pneumothorax, retinal detachment, scoliosis, sleep apnea, stretch marks in pregnancy, teeth crowded, narrow and thin face, and temporomandibular joint dysfunction [11-13]. Diagnosis of MFS is often based on the Ghent criteria, family history, and genetic testing (DNA analysis) [10]. Weill-Marchesani syndrome, Loeys-Dietz syndrome, and Ehlers- Danlos syndrome are considered in differential diagnosis [10].
There is no cure for Marfan syndrome, but an accurate and prompt diagnosis is pivotal to providing the best treatment to patients as early as possible [10]. Heterotaxy syndromes refer to abnormal left/right distribution of thoracic and abdominal organs that is neither situs solitus nor situs inversus. They are commonly associated with congenital heart disease (CHD) and visceral malformations. Isomerism applied in this syndrome as mirrored organs. There is either left or right isomerism also present in Heterotaxy syndromes. Right isomerism is identified as asplenia syndrome. There are associated severe cyanotic CHD, absence of the spleen, bilateral eparterial bronchi, bilateral trilobed lungs, bilateral right atria, midline/transverse liver, and intestinal malrotation. Left isomerism is identified as polysplenia syndrome. There are associated multiple splenules without a parent spleen azygos or hemiazygos continuation of the inferior vena cava, bilateral hyparterial bronchi, bilateral bilobed lungs, bilateral pulmonary/ left atria, midline/transverse liver, and intestinal malrotation [14].
In this manuscript, I reported a case of an adolescent singlestudent male patient was presented to the intensive care unit (ICU) with angina and alternation of the chest pain referral to both arms (Figure 1). So, how would you manage this case?
Figure 1:Graphical abstract showed diagrammatic presentation of Right to Left Angina Yasser’s Syndrome (Swinging Yasser’s Central Heart Syndrome) or Dancing Yasser’s Heart Syndrome.
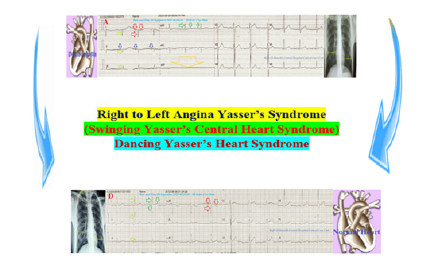
Case Presentation
A 17-year-old adolescent single-student male patient was presented to the intensive care unit (ICU) with angina. Insomnia, night leg movements, and blurred vision were associated symptoms. Currently, he has a recent history of intermittent mild angina with alternation of chest pain referral to both arms. The mother gives a history of at-home and outside movement hyperactivity. Upon general physical examination, generally, the patient appeared long, thin, and hyperactive, with a regular pulse rate (VR of 80), blood pressure (BP) of 130/70mmHg, respiratory rate of 16bpm, a temperature of 36 °C, pulse oximeter of oxygen (O2) saturation of 98%, and GCS of 15/15. There is inferior eye lens dislocation (Figure 2A), high deep upper high arched palate (Figure 2B), malformed teeth (Figure 2C), curling dark black hair, abnormal external ears (Figure 2D), thin long spider-shape fingers (Figure 2E), café au late patches (Figure 2F), abnormal right leg movements, and bilateral bifid internal malleoli (Figure 2G). Tests for provocative latent tetany were positive. There are no local cardiorespiratory findings and no more relevant clinical data were noted during the clinical examination. The patient was admitted to ICU with angina for follow-up for a few hours with no treatment. The initial ECG tracings were done during the initial presentation with right angina in the ICU showing NSR (VR of 81, 82, and 82), RAD, global negative deflections in both I and aVR leads, inverted T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is stunted (Figures 3A-C).
Figure 2:Different images for the patient were taken on the initial presentation; inferior eye lens dislocation (A) high arched palate (B), malformed teeth (C), curling dark black hair, abnormal external ears (D), thin long spider-shape fingers (E), café au late patch (F), abnormal right leg movements, and bilateral bifid internal malleoli (G).

Figure 3A-C:Serial ECG tracings; A-C tracings were done during the initial presentation with right angina in the ICU showing NSR (VR of 81, 82, and 82), RAD (lime arrows), global negative deflections in both I (red arrows) and aVR leads (green arrows), inverted T-waves in lead II (dark blue arrows) with normal R-wave progression in V1-6 leads. An aVF lead is stunted (large golden arrow).
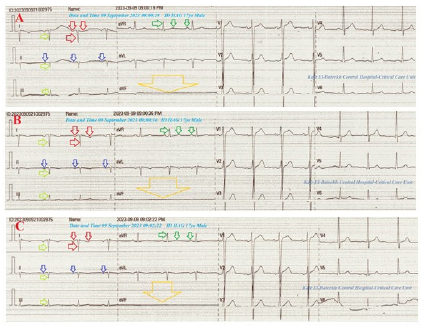
The second group of ECG tracings was taken within 22minutes of the above ECG tracings during left angina and after changing the above electrocardiograph apparatus showing NSR (VR of 72 and 82), normal axis, global positive deflections in both I and aVR leads, reversed T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is normalized (Figures 3D&E). The third group was ECG tracing taken within 30 minutes of the above ECG tracings during recurrent right angina showing NSR (VR of 78), right axis deviation, global negative deflections in both I and aVR leads, inverted T-waves in lead II with normal R-wave progression in V1-6 leads. The lead II is stunted (Figure 3F).
Figure 3D-E:Tracings were done within 22minutes of the above ECG tracings during left angina after changing the above electrocardiograph showing NSR (VR of 72 and 82), normal axis (lime arrows), global positive deflections in both I (green arrows) and aVR leads (red arrows), reversed T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is normalized.
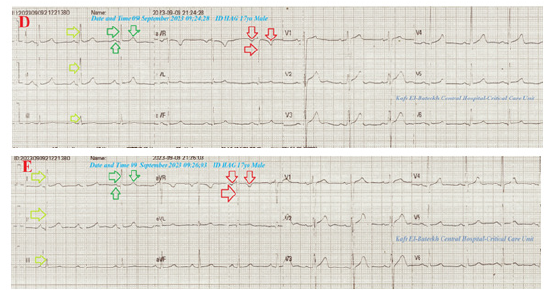
Figure 3F:Graphical abstract showed diagrammatic presentation of Right to Left Angina Yasser’s Syndrome (Swinging Yasser’s Central Heart Syndrome) or Dancing Yasser’s Heart Syndrome.

The fourth group was ECG tracing taken within 2 minutes of the above ECG tracings during recurrent right angina showing NSR (VR of 73), RAD, global negative deflections in both I and aVR leads, inverted T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is stunted (Figure 3G). The fifth group was ECG tracing taken on the second day of the above ECG tracings with no pain showing NSR (VR of 65), normal axis, global positive deflections in I, global negative deflections aVR leads, reversed T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is normalized (Figure 3H). The sixth group was ECG tracings taken within 7 days of the above ECG tracings with no pain showing NSR (VR of 70 and 71) with normal axis, muscle spikes artifacts, and their disappearance (Figures 3I&J). The initial complete blood count (CBC); Hb was 13.1g/dl, RBCs; 4.85*103/mm3, WBCs; 3*103/ mm3 (Neutrophils; 42.5%, Lymphocytes: 50.4%, Monocytes; 7.1%), Platelets; 197*103/mm3. SGPT was (20U/L) and SGOT was (13 U/L). The serum albumen was (4.5mg/dl).
Figure 3G:Tracing done within 2 minutes of the above ECG tracings during recurrent right angina showing NSR (VR of 73), RAD (lime arrows), global negative deflections in both I (red arrows) and aVR leads (green arrows), inverted T-waves in lead II (dark blue arrows) with normal R-wave progression in V1-6 leads. An aVF lead is stunted (large golden arrow).
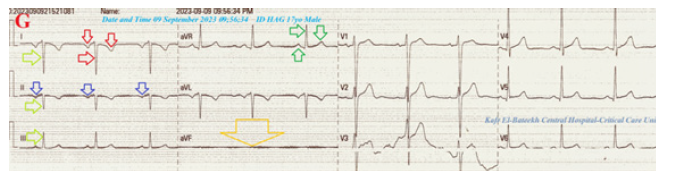
Figure 3H:Tracing was done on the second day of the above ECG tracings with no pain showing NSR (VR of 65), normal axis (lime arrows), global positive deflections in I (green arrows), global negative deflections aVR leads (red arrows), reversed T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is normalized.

Figure 3:I. Tracing was done within 7 days of the above ECG tracings with no pain showing NSR (VR of 70) with normal axis and muscle spikes artifacts (golden arrows). J. Tracing was done within 22 seconds of the I. tracing with no pain showing NSR (VR of 71) and disappearance of the above muscle spikes artifacts.

Figure 4:Chest XR-PA view A-film was done on the initial presentation and during right angina showing a more centralized heart with nearly equal right and left cardiothoracic distance (lime rectangles and blue lines), thoracic spines more centered the heart (golden arrow), and the heart is perpendicular on round equal both right and left diaphragmatic copula (red circle). B-film was done on the second day of the presentation and there was no angina showing more deviated heart to the left with righter than left cardiothoracic distance (lime rectangles and blue lines), thoracic spines more to the right the heart (golden arrow), and the heart is in diagonal on more elevated the right than the left diaphragmatic copula (red circle).
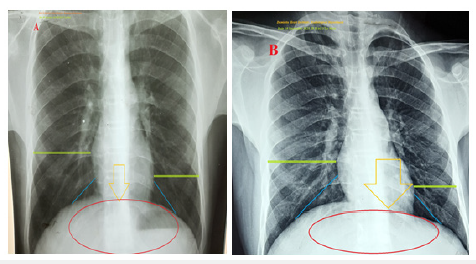
Serum creatinine was (0.6mg/dl). RBS was (88mg/dl). Total calcium was (7.09mg/d). Ionized calcium was (3.7mg/d). The troponin I tested was negative. The CK-MB was (11 U/L). Chest XRPA view film was done on the initial presentation and during right angina showing a more centralized heart with nearly equal right and left cardiothoracic distance, thoracic spines more centered the heart, and the heart is perpendicular on round equal both right and left diaphragmatic copula (Figure 4A). The second chest XR-PA view film was done on the second day of the presentation and there was no angina showing more deviated heart to the left with righter than left cardiothoracic distance, thoracic spines more to the right heart, and the heart is in diagonal on more elevated the right than the left diaphragmatic copula (Figure 4B).
Echocardiography was done on the second day of the presentation showing a normal aortic valve, a mild tricuspid regurgitation, and trivial mitral regurgitation of an EF of 67% (Figure 5). Abdominal ultrasound is normal with all organs and viscera in place. Right to left angina Yasser’s syndrome (Swinging Yasser’s central heart syndrome) or Dancing Yasser’s heart syndrome was the most probable diagnosis. Within 6 hours of monitoring and follow-up, the patient was finally discharged with further recommended cardiac follow-up.
Figure 5:Echocardiography was done on the second day of the presentation showing a normal aortic valve (red arrow) a mild tricuspid regurgitation (lime arrow) and trivial mitral regurgitation (golden arrow) of an EF of 67% (yellow arrow).

Discussion
Overview
An adolescent single-student male patient was presented to the critical care unit with alternating angina in referral to either right or left arms.
The primary objective for my case study was the presence of an adolescent single student male patient who was presented to the critical care unit with alternating angina in referral to either right or left arms in the ICU.
The secondary objective for my case study was the question of; How did you manage the case? There was a history of alternating angina in referral to either right or left arms. The presence of right angina during the initial presentation with initial ECG tracings showing RAD, global negative deflections in both I and aVR leads, inverted T-waves in lead II (Figures 3A-C) is parallel to the ECG of dextrocardia. However, the normal R-wave progression in V1-6 leads and stunted aVF lead are against the diagnosis of dextrocardia. The initial CXR-PA view film presentation during right angina shows a more centralized heart with nearly equal right and left cardiothoracic distance, thoracic spines more centered the heart, and the heart is perpendicular on round equal both right and left diaphragmatic copula (Figure 4A) is correlating with ECG changes. Spontaneously, within 22 minutes of the above ECG tracings during left angina and after change the above electrocardiograph apparatus showed normal axis, global positive deflections in both I and aVR leads, reversed T-waves in lead II with normal R-wave progression in V1-6 leads. An aVF lead is normalized (Figure 3D&E). These changes indicate a normal ECG.
The initial CXR-PA view film presentation on the second day of the presentation and there was no angina showing more deviated heart to the left with righter than left cardiothoracic distance, thoracic spines more to the right heart, and the heart is in diagonal on more elevated the right than the left diaphragmatic copula (Figure 4B). Recurrence of ECG of the above changes during alternation of right and left angina had happened but the stunted limb was II (Figures 3F&G). Appearance of the muscle spike artifacts may be interpreted as hyperactivity, anxiety, and more active abnormal leg movements (Figure 3I). There are some similarities findings to MFS such as high-arched palate, malformed teeth, palpitations, tall, thin body, long arms, legs, fingers, and toes, and bilateral bifid medial malleoli (Figures 2B, C, E&G). But there are some dissimilarities findings to MFS such as curling dark black hair, prominent ear, inferior lens dislocation, café au late patch, abnormal rest leg movements (Figures 2C, D&F), hyperactivity, and insomnia. Heterotaxy syndromes with either left or right isomerism are also implicated in the differential diagnosis of the current case. But there is no clinical correlation with the case [14]. The parallel changes during right angina with ECG nearly dextrocardia and centralized heart in CXR film are more interesting (Figures 3A-C,4A). Also, the reversed changes during left angina with normalized or standard ECG and non-centralized heart to more left in the CXR film are more motivating (Figures 3A-C,4A). Despite there being no exact explanation for the last two points’ changes, dancing hyperactivity cardiac movements sometimes to right and sometimes to left in incomplete circus fashion may be interpretative theory. The traction theory may help the interpretation of nearly equal elevated diaphragmatic copulas in the centralized heart in the CXR film during right angina (Figure 4A). The opposite happened with a non-centralized heart to more left in the CXR film (Figure 4B).
Although there is clinical evidence of alternating angina no ECG, echocardiographic (Figure 5). However, the twisting theory for coronary arteries during dancing cardiac movements may explain this angina. I can’t compare the current case with similar conditions. There are no similar or known cases with the same management for near comparison. The only limitation of the current study was the expensive costs of genetic and karyotyping analysis.
Conclusion and Recommendations
Right to left angina Yasser’s syndrome (Swinging Yasser’s central heart syndrome) or Dancing Yasser’s heart syndrome is a new and innovative cardiovascular syndrome. Due to some similarities, Marfan syndrome, dextrocardia, and Heterotaxy syndromes were implicated in differentiation. Dancing hyperactivity, traction, and twisting theories are interpretative suggested theories for this new syndrome.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I wish to thank my wife for saving time and improving the conditions for helping me.
References
- Moore KL, Dalley AF, Agur AMR (2009) Clinically oriented anatomy. Journal of Anatomy 215(4): 474.
- Knipe H (2023) Cardiac position. Radiopaedia, Australia.
- Kumar S, Nandyal SR, Kaushik S, Ibrahim S (2022) Rare case of mesocardia with ostium secundum ASD and double IVC. BMJ Case Report 15(4): e249263.
- Stephenson, Susan R (2012) Diagnostic medical sonography obstetrics and gynecology. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA.
- Leung AK, Robson WL (2006) Dextrocardia with situs [corrected] solitus. CMAJ 175(3): 244.
- Bindra S, Tabibiazar R, Mazar M, Dave R (2011) ECG leads must be placed in reversed positions on a person with dextrocardia. Proceedings of UCLA Healthcare UCLA 15: 1-3.
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, et al. (2010) The revised Ghent nosology for the Marfan syndrome. Journal of Medical Genetics 47(7): 476-485.
- Nistri S, Cario RD, Sticchi E, Spaziani G, Della MM, et al. (2021) Differential diagnosis between Marfan syndrome and loeys-dietz syndrome type 4: A novel chromosomal deletion covering TGFB2. Genes 12(10): 1462.
- NHLBI, NIH (2010) What is marfan syndrome? The National Heart, Lung, and Blood Institute, Bethesda, Maryland, USA.
- Marelli S, Micaglio E, Taurino J, Salvi P, Rurali E, et al. (2023) Marfan syndrome: Enhanced diagnostic tools and follow-up management strategies. Diagnostics (Basel) 13(13): 2284.
- Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM (1995) Marfan syndrome: Long-term survival and complications after aortic aneurysm repair. Circulation 91(3): 728-733.
- Fitzgibbons RJ, Forse RA (2015) Clinical practice: Groin hernias in adults. The New England Journal of Medicine 372(8): 756-63.
- Kohlmeier L, Gasner C, Bachrach LK, Marcus R (1995) The bone mineral status of patients with Marfan syndrome. Journal of Bone & Mineral Research 10(10): 1550-1555.
- Weerakkody Y, Sharma R, El-Feky M (2023) Heterotaxy syndrome. Radiopaedia, Australia.
© 2024 Yasser Mohammed Hassanain Elsayed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






