- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Long-Standing Severe Supravalvular Aortic Stenosis: Heart Response and Adaptation
M Griselli1*, C Montanaro2# and DF Shore2#
1Department of Cardiac Surgery, King Abdullah bin Abdulaziz University Hospital, Riyadh, Saudia Arabia
2Department of Cardiac Surgery, Royal Brompton Hospital, London, United Kingdom
#These authors contributed equally to this work and share first authorship
*Corresponding author: Massimo Griselli, Department of Cardiac Surgery, King Abdullah bin Abdulaziz University Hospital, Riyadh, Saudia Arabia
Submission: November 27, 2023;Published: December 14, 2023

ISSN 2578-0204Volume4 Issue3
Abstract
Supravalvular aortic stenosis (SAS) is a heart defect that develops before birth. This defect is a narrowing (stenosis) of the aorta and the condition is described as supravalvular because the section of the aorta that is narrowed is located just above the aortic valve. SAS represents 8% to 14% of all congenital aortic stenosis. SAS has been classified in various ways: hourglass, membranous, and hypoplasia of aortic arch or more commonly in discrete or diffuse. Hourglass or discrete type SAS is the most common [1,2]. Some people with SAS also have defects in other blood vessels, most commonly stenosis of the pulmonary arteries. An abnormal heart sound during a heartbeat (heart murmur) can often be heard during a chest exam. If SAS is not treated, the aortic narrowing can lead to shortness of breath, chest pain, and ultimately heart failure. The severity of SAS varies considerably, even among family members. Some affected individuals need treatment in infancy, while others never experience symptoms of the disorder.
Keywords:Aorta; Supravalvar; Hypertrophy
Introduction
Supravalvular aortic stenosis is a rare anomaly of left ventricular outflow tract obstruction with an estimated incidence of 1:20000 live births. SAS is a generalized disease of the arterial wall resulting in an exaggerated narrowing of the level of sinu-tubular junction (STJ) caused by severe thickening of the media and the intima layers. SAS is very often part of the Williams- Beuren (WBS) syndrome and can be associated with involvement of pulmonary artery tree and other arteries in the body.
WSB is a rare genetic disorder with facial features including a broad forehead, underdeveloped chin, short nose, and full cheeks, mild to moderate intellectual disability with particular challenges with visual spatial tasks such as drawing. Many people with WS have an outgoing personality, an openness to engaging with other people, and a happy disposition. However, SAS can occur in non-syndromic conditions as well in conjunction with other left sided obstructive lesions or as isolated lesion. SAS in WBS and in non-syndromic cases is associated with systemic elastin arteriopathy, a genetic disease inherited as autosomal dominant disease with incomplete penetrance a variable expressivity. The result is a lack of elastin continent in the vessels affected exposing them to progressive thickening and fibrosis.
This process at the STJ causes a drawing of commissures together reducing the space between the aortic valve leaflets and the STJ. The thickening process may extend to the coronary ostia with consequent narrowing and in the extreme forms may involve all the aorta with a possible involvement of head and neck vessels origin. SAS determines left ventricular hypertrophy (LVH) and impairment of coronary blood flow due to limited space between aortic valve leaflets and STJ, possible narrowing of coronary ostia and increased myocardial demands secondary to the LVH. Therefore, coronary events as sudden cardiac arrest or ventricular arrhythmias may happen because of reduced coronary blood flow. Symptoms develop late in the clinical course, again mainly related to a restricted coronary flow causing angina and sudden cardiac arrest. SAS is easily diagnosed with trans-thoracic echocardiography and magnetic resonance (MR) imaging and computerized tomography (CT) imaging is used to assess accurately all arterial trees particularly when planning surgical procedures. Because of the progression of SAS in childhood, surgery is offered before severe LVH develops and various surgical techniques have been used successfully over the years whilst little data is available on outcomes in the adult population. We would like to report a case of severe SAS in a 17-year-old lady where extreme pathophysiological responses and adaptation from the heart and coronary arteries were found.
Clinical Case
Our patient was presented to her local hospital with symptoms of chest pain, breathlessness, and dizziness. Initial trans-thoracic echocardiography showed supravalvular aortic stenosis and therefore was transferred to our institution for further management. Her background included ‘murmur at birth’ not followed up, mild iron deficiency anemia and macrothrombocytopenia and mild anxiety. She reported a 3-month history of progressive exertional breathlessness, noticing that the stairs at school became more difficult and now struggling to do even 1 flight of stairs at home. Recently chest pain and intermittent palpitations became more common and the family doctor picked up a murmur and referred her for investigations as an out-patient, but she was admitted to the local hospital because of worsening symptoms. On examination at arrival, she had small teeth but not widely spaced without obvious facial features of Williams syndrome, slow rising pulse, thrill in the left upper sternal edge and loud ejection systolic murmur over the precordium radiating into the back.
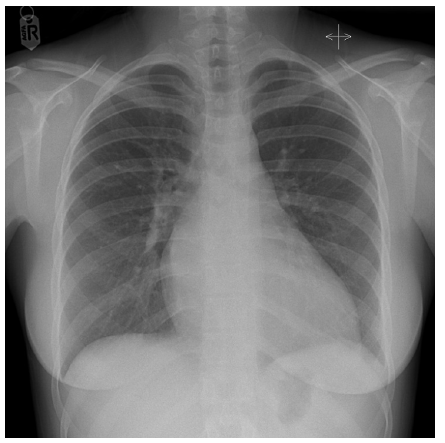
The chest was clear, jugular venous pressure not raised, no peripheral oedema, abdomen soft and non-tender, no liver edge, systolic blood pressure 97/46 with heart rate of 70/minute. Electrocardiography showed sinus bradycardia with features of severe LVH (Figure 1), and chest X-ray showed mild cardiomegaly (Figure 2).
Figure 1:12-lead ECG showing sinus rhythm, 62bpm, normal atrio-ventricular conduction, signs of LVH, normal QTc.
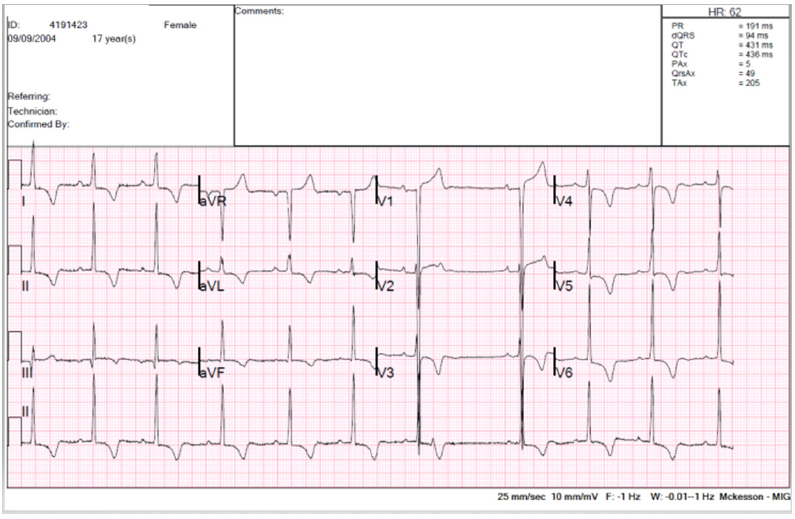
Figure 2:Chest X-Ray, showing situs solitus, levocardia, left sided aortic arch. Small right-side bulging of the aorta. Cardiomegaly. Indistinct pulmonary artery branches.
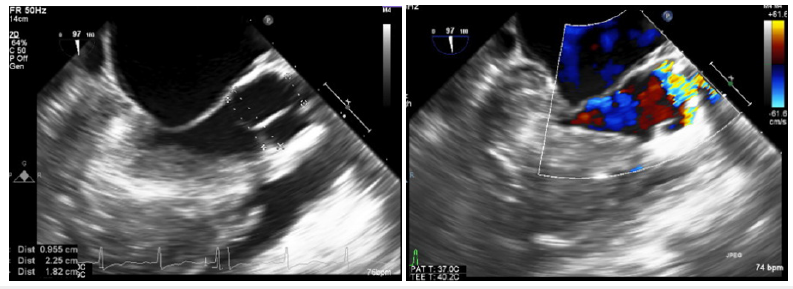
Trans-thoracic echocardiography (TTE) (Figure 3) and a more detailed trans-esophageal echocardiography (TEE) (Figure 4) at the time of surgery showed normal size left ventricle (LV) with hyperdynamic ejection fraction of 75-80% and reduced longitudinal function. Severe LVH with LV intracavity obstruction; severe supravalvular aortic stenosis with a minimum diameter of 10mm at the level of the STJ at 16mm from the aortic valve annulus; the aortic valve annulus measure was of 18 mm and the aorta at the sinuses of Valsalva level measured 2.2cm. in diameter and both coronary arteries origins were very dilated. The systolic gradient across the SAS was about 130mmHg.
Figure 3:Transthoracic echocardiogram, parasternal short axis showing concentric LVH (a). Transthoracic echocardiogram, 4-chamber view showing biventricular small cavity, concentric biventricular hypertrophy and loss of apical tapering (b).
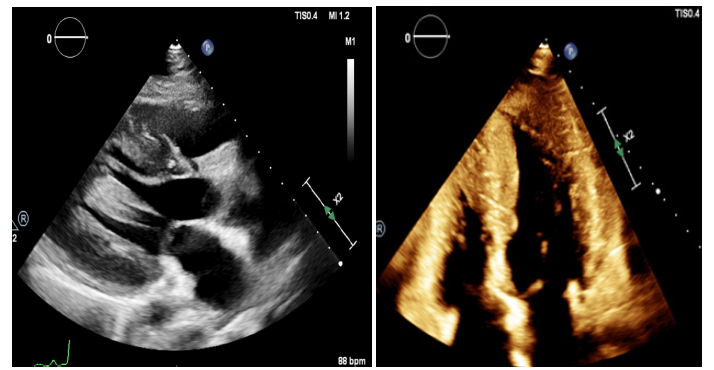
Figure 4:Transesophageal echocardiogram, outflow tract view, systole. Severe supravalvular aortic stenosis with a minimum diameter of 10mm at the level of the sino-tubular junction at 16mm from the aortic valve annulus; the aortic valve annulus measured was of 18mm and the aorta at the sinuses of Valsalva level measured 22mm. The systolic gradient across the SAS was about 130mmHg. There is acceleration flow only at the level of the sino-tubular junction (STJ) and not at valve level (AV).s
Cardiac MR (Figure 5) and CT angiography (Figure 6) were performed at our Institution. MR angiography confirmed the severity of the LVH and SAS, the aneurysmatic very dilated origin of coronary arteries but also showed a patchy and diffuse subendocardial enhancement from mid cavity to the apex of LV. This was probably caused by myocardial infarction secondary to a vasculitis process given the appearance of coronary arteries. MR imaging of other arterial trees like renal or cerebral were normal except for some tortuosity of carotid and vertebral arteries. At surgery the ascending aorta was opened longitudinally down towards the non-coronary sinus. The narrow part above the aorta was even smaller than the echocardiography showed (around 7-8mm) and there was dense fibrous tissue with calcifications involving both commissures supporting the left coronary leaflet. The commissures were shaved of calcified fibrous tissue and all the narrow area was then patched with a generous bovine pericardial patch as single patch technique. We could appreciate the severe hypertrophy of the left ventricle with a very small tight cavity and the size of coronary ostia with the left one of almost 2cm without any kind narrowing seen. The post-surgery TEE showed a good repair of SAS with a residual systolic gradient of 35mmHg. The patient was discharged uneventfully from our hospital on day 6 post-surgery on Aspirin 80mg daily.
Figure 5:Cardiac Magnetic Image. Short axis view is diasole (D) and systole (S) showing the severe LVH and systolic mid-cavity obliteration as result of the severe supravalvar stenosis (SAS). The hypoplastic ascending thoracic aorta is also noted (5a). The patchy subendocardial late gadolinium enhancement (white arrows) is noted in the long-axis two and four chamber and it is compared to the correspondent cine acquisition.
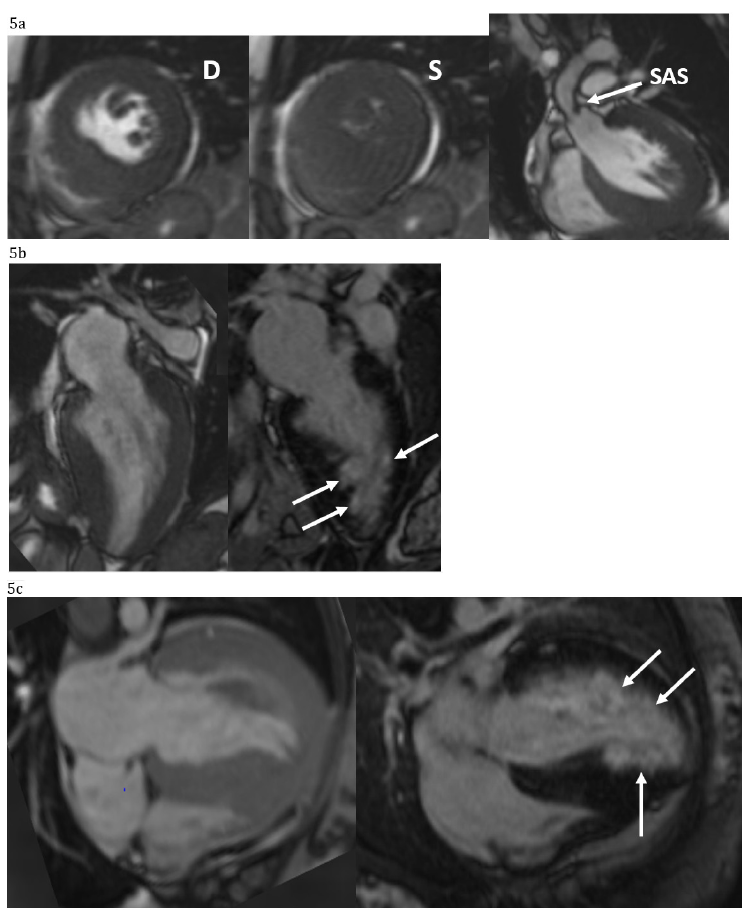
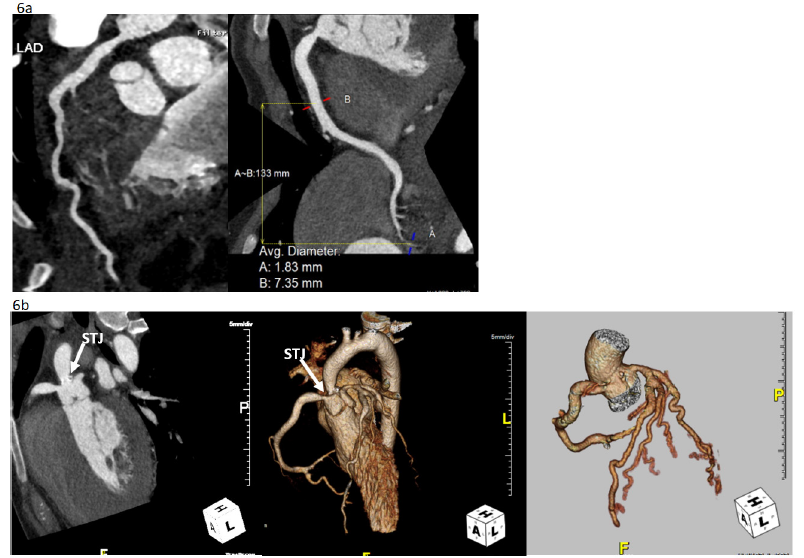
Figure 6:Cardiac CT showing coronary arteries diffusely enlarged (max 7mm at proximal RCA) without focal aneurysm formation (a). There is a close relation between the severely narrowed sino-tubular junction (STJ), but this does not directly involve the coronary ostia. There is hypoplasia of the ascending thoracic aorta and of the proximal and mid left-sided aortic arch (b).
Discussion
We are delighted to present this case as we found several points which need to be discussed in this section. We know that LVH is the ‘natural’ response to pressure load caused by the left ventricular outflow tract obstruction of any type. The extent and severity of LVH has two consequences: development of cardiac failure due to a restriction in the cardiac output and increased myocardial metabolic demand due to imbalance between the hypertrophied ventricular mass and the coronary blood flow. In our patient, evidence of cardiac failure developed before symptoms of coronary hypoperfusion were reported. This may be explained by the fact that coronary hypoperfusion became evident only when the cardiac output decreased. Before that time, despite the severe LVH, no chest pain or palpitations were reported by the patient. Anatomically we know that the coronary ostia and part of the coronary arteries were massively dilated in response to the proximal pressure load, causing extreme sheer stress to the genetical abnormal tissue of the aortic root. However, the coronary arteries in this case did not have any narrowing at any level and the distance between the aortic valve leaflets and the STJ were not too short to jeopardize the coronary flow resulting from limited clearance between aortic valve and STJ. Therefore, we believe that development of heart failure was the primary event in this lady which in turn tilted the already precarious balance between myocardial demand and coronary flow causing classical ‘coronary’ insufficiency symptoms. We are also surprised by the length of time that this went along without symptoms considering the severity of SAS. We know that SAS progresses mainly during childhood and much less during adulthood, so we believe that the level of obstruction has been present for quite a long time without causing any symptoms.
Conclusion
Our case shows the extreme adaptation of the heart to a long-standing tight SAS represented by the severity of LVH and the dilation of the proximal part of the coronary artery course. Our patient was seen recently in an out-patient clinic, and she is asymptomatic. The echocardiography showed a good ventricular function with unchanged low gradient (~30mmHg) across the left ventricular outflow tract without evidence of aortic regurgitation and she remained on Aspirin from the hospital discharge. Regarding the follow-up on these patients, quite interesting are the conclusions from Roemers et al. In their report over 52 years’ experience on outcome regarding surgical correction of SAS, they showed that the survival of SAS patients is much lower compared to the survival in the general population, making the SAS not a benign disease and patients should be followed up closely for the rest of their lives.
Furthermore, in their 4 of the six late deaths were out-ofhospital cardiac arrests at an age between 33 and 57 years, all operated at a relatively old age (>14). They hypothesized that the coronary arteries were exposed to a high pressure in the pre-stenotic area for a relatively long period of time, which might have caused endothelial damage leading to ischemic heart disease responsible for their sudden cardiac arrest. [3]. To reinforce the above concept of a not benign disease we have this report from greutmann et al. [4]. In their multicenter retrospective study, cardiac outcomes were assessed for adult patients (≥18 years) with SAS, and they looked at the adverse cardiac events (cardiovascular death, myocardial infarction, stroke, heart failure, sustained arrhythmias, and infective endocarditis) and the need for cardiac surgery. They showed that although the progression of SAS in adulthood is rare, adults with SAS remain at risk for cardiac complications and reoperation, with valve surgery the most common indication [4]. However, in current practice, nowadays, results surgical repair of SAS have a good long-term outcome (Survival 90%±7%, 84%±9%, and 8%2±10% at 5, 10, and 20 years, respectively) with late mortality and need for reoperation are more likely with diffuse SAS or the presence of aortic valve stenosis (2). Another study reported a cumulative survival for all patients was 96% at 5 and 10 years with good outcome with any surgical technique [5].
However, the Stanford group with Mcelhinney et al. [6] in patients with SAS, abnormalities of the aortic valve, subaortic region, and coronary arteries are often present and their management is as critical to the long-term outcome of these patients as reconstruction of the aorta, including aggressive valvuloplasty which could decrease the incidence of subsequent aortic valve replacement, choosing the Ross procedure as the preferred approach in some patients with complex outflow tract obstruction.
References
- Vindhyal MR, Priyadarshni S, Eid F (2023) Supravalvar aortic stenosis. In: StatPearls. Treasure Island, StatPearls Publishing, Florida, USA, p. 25.
- Deo SV, Burkhart HM, Schaff HV, Li Z, Stensrud PE, et al. (2012) Late outcomes for surgical repair of supravalvar aortic stenosis. Ann Thorac Surg 94(3): 854-859.
- Roemers R, Kluin J, De Heer F, Arrigoni S, Bovenkamp R, et al. (2018) Surgical correction of supravalvar aortic stenosis: 52 years' experience. World J Pediatr Congenit Heart Surg 9(2): 131-138.
- Greutmann M, Tobler D, Sharma NC, Vonder Muhll I, Mebus S, et al. (2012) Cardiac outcomes in adults with supravalvar aortic stenosis. Eur Heart J 33(19): 2442-2450.
- Scott DJ, Campbell DN, Clarke DR, Goldberg SP, Karlin DR, et al. (2009) Twenty-year surgical experience with congenital supravalvar aortic stenosis. Ann Thorac Surg 87(5): 1501-1507.
- McElhinney DB, Petrossian E, Tworetzky W, Silverman NH, Hanley FL, et al. (2000) Issues and outcomes in the management of supravalvar aortic stenosis. Ann Thorac Surg 69(2): 562-567.
© 2023 M Griselli. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






