- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Anemia Caused by Erythropoietin Stimulating Agent in End-Stage Kidney Disease Patient
Magbri A*, El-Magbri M and Hernandez PA
Marshfield Medical Center, USA
*Corresponding author: Magbri A, Marshfield Medical Center, Weston, USA
Submission: November 16, 2022;Published: January 26, 2023

ISSN 2578-0204Volume4 Issue2
Abstract
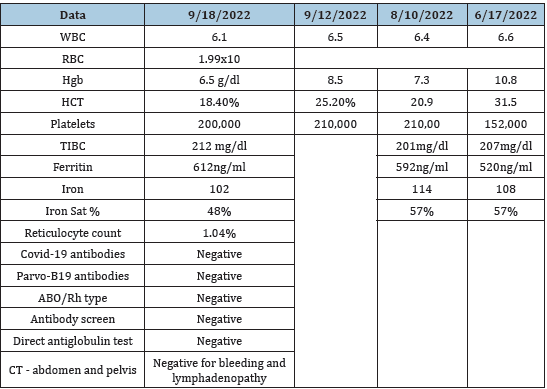
We reported a case of severe anemia in a patient with End-Stage Kidney Disease (ESKD) on dialysis. The anemia developed when the patient is switched from hemodialysis (HD) to Peritoneal Dialysis (PD) when the intra-venous Erythropoietin Stimulating Agent (ESA, Epogen) was changed into subcutaneous injection of darbepoetin. The patient’s hemoglobin dropped 2 grams in about two months during this period (Table 1). Extensive work-up including, bleeding disorders, hemolysis, iron deficiency, infections including CMV, Epstein-Bar virus, parvo-19 virus infection were unrevealing (Table 1). The anti-Epogen neutralizing antibodies were not measured due to unavailability. Bone marrow biopsy and aspirate were negative for infiltrations or Myelodysplastic Syndrome (MDS). The leukocyte and platelet counts were normal. Even though anti-ESA antibodies were not measured in this case, all tentative causes of his anemia were excluded by laboratory investigations. The patient’s anemia was treated symptomatically with blood transfusion and discontinuation of the ESA treatment. He made a remarkable recovery.
Table 1
Keywords: Pure red cell aplasia (PRCA); ESKD (end-stage kidney disease); Hemodialysis; Peritoneal dialysis; Bone marrow aspirate and biopsy; Anti-EPO antibodies; Neutralizing antibodies; Myelodysplastic syndrome
Abbreviations:WBC: White Blood Cell Count; RBC: Red Blood Cell Count; Hgb: Hemoglobin; HCT: Hematocrit; TIBC: Total Iron Binding Capacity; Ng/Ml: Nanogram Per Milliliter
Introduction
Pure Red Cell Aplasia (PRCA) is a syndrome characterized by a normocytic normochromic anemia with severe reticulocytopenia and marked reduction in the erythroid precursors in the bone marrow. The white blood cell and the platelet counts are usually normal. Acquired PRCA is a primary disorder or secondary to some other causes. The primary acquired PRCA is an autoimmune disorder that is antibody mediated. Many cases of acquired PRCA are idiopathic. Secondary acquired PRCA may be associated with collagen vascular/ autoimmune diseases such as systemic lupus erythematosus; lymphoproliferative disorders such as chronic lymphatic leukemia or lymphoma; infections, particularly with parvovirus B19; thymoma and other solid tumors. Other toxins and drugs are sometimes implicated as a cause of PRCA, like chloramphenicol, rifampicin, phenytoin, azathioprine, and isoniazid [1]. PRCA has been described in patients treated with epoetin and other Erythropoiesis Stimulating Agents (ESAs), stemming from the induction of neutralizing antibodies directed against the ESA molecule [2].
Most reported cases have been in patients receiving ESA for chronic kidney disease- related anemia. The aim of presenting this case is to draw attention to the possibility that rare causes of anemia in patients on dialysis must be considered and ruled out before embarking on symptomatic treatment. Myelodysplastic syndrome can uncommonly be presented as PRCA and must be excluded by bone marrow aspirate and flow cytometry. Cyclosporin, with or without corticosteroids, appears to be the single most effective immunosuppressive agent used in the treatment of PRCA.
Case History
A 63-year-old Caucasian male, with past medical history significant for hypertension, T2DM, stroke, and severe anemia. His anemia was responsive to intravenous Epogen when he was started on hemodialysis (HD). Then he developed a precipitous decrease in his hemoglobin over a period of eight weeks. His reticulocyte count was low, and his iron stores were normal. The abrupt decrease in his hemoglobin occurred when darbepoetin is switched to subcutaneous injection for his anemia treatment (Table 1). His physical examination revealed an obese man with no significant shortness of breath, alert and oriented x 3. His heart, chest and abdominal examination were unremarkable. He has mild lower extremities edema. He was started on HD for his ESKD in May 2022 via a tunnel dialysis catheter in the right internal jugular vein. He then switched to Peritoneal Dialysis (PD) in June 2022. He was on Epogen intravenous injection while he was on HD. When he was switched to PD, his Epogen is replaced by Darbepoetin subcutaneously.
A CT scan of the abdomen and pelvis failed to show any retroperitoneal bleeding or lymphadenopathy. During the interim time he received blood transfusion to maintain his HgB in the normal range. His hemoglobin dropped from 10.8 to 6.5g/dl in 8-12 weeks, (Table 1). Despite extensive work-up for bleeding including upper and lower endoscopy and screen for infection, no cause was found to account for his anemia (Table 1). There was no evidence of hemolysis to explain his precipitous decline in the HgB (Table 1). His bone marrow examination revealed normal WBC and platelets precursors with marked reduction of the red blood cell precursors No evidence of bone marrow infiltration was found, pictures is not available. Although, the anti-EPO antibodies were not measured due to unavailability. Given the clinical pictures and the fact that he has developed only anemia with normal iron stores, white cell count, and platelet count, marked reduction of the reticulocyte count and with no evidence of bone marrow disease, PRCA was considered the plausible cause of his anemia. No immunosuppression was started based on the recommendation of the hematologist. The patient’s anemia was treated symptomatically with blood transfusion and discontinuation of the ESA treatment. He made a remarkable recovery.
Discussion
The diagnosis of PRCA rests on excluding other causes of normocytic normochromic anemia with normal white cell and platelets counts. In PRCA, the reticulocyte count and the reticulocyte index should be low with marked reduction in the red cell precursors in the bone marrow, in a patient who received ESA injections for his/her anemia. Most cases of Non-Erythropoietin (EPO) related PRCA are mediated by IgG autoantibodies or cytotoxic T lymphocytes directed against erythroid precursor or progenitor cells [1,3]. PRCA against endogenous EPO is rare in patients who have never been treated with ESA [4-6]. Several preparations of ESA produced by various manufactures differ from each other by the degree of glycosylation and sialic acid content [7]. The vast majority of PRCA occurred in patients who are treated with epoetin alfa (Eprex, in single-use syringes) which are used outside the United States. EPO-related PRCA has been described with other preparations including darbepoetin alfa and methoxy polyethylene glycol-epoetin beta [8,9].
Almost all reported cases of anti-EPO antibody-mediated PRCA have occurred in patients with Chronic Kidney Disease (CKD) who have received the drug subcutaneously [2,10-12]. Reports of anti- EPO antibody mediated PRCA have occurred in patients in Canada, Australia, and Asia [11,13]. The condition remains extremely rare, given the widespread use of EPO and other ESA in treating patients with anemia of chronic disease [14,15]. The reported incidence of PRCA occurring with subcutaneous exposure of ESA is estimated to be 1.6 per 10,000 patient-years [16]. There have been over 200 reported cases of PRCA cases caused by Eprex use representing most reported PRCA cases [11,13,17]. Fortunately, EPO PRCA has become exceedingly rare after the modification of the EPO backages [18]. Development of anti-EPO non-neutralizing and neutralizing antibodies has occurred in Thailand related to subcutaneous use of product manufactured and used outside the United States [19- 22]. Based on Canadian studies the routine screening for anti-EPO antibodies cannot be justified given the rarity of the conditions [23,24].
The 2012 kidney Disease: Improving Global Outcome (KDOGO)
guidelines, suggest evaluation for PRCA due to anti-EPO antibodies
should occur in a patient exposed to at least eight-weeks of ESA
therapy who develops all the following [25]:
A. Decrease in hemoglobin (Hb) level of >0.5 to 1 g/dl per
week or transfusion requirement of at least one to two units of
PRBCs to maintain adequate Hb.
B. Normal platelet and White Blood Cell (WBC) count.
C. Absolute reticulocyte count of <10,000/mcL.
D. Elevated serum transferrin saturation and serum ferritin,
reflecting decrease utilization of iron secondary to diminished
erythrocytosis which can be a clue to the occurrence of PRCA.
E. Evaluation of PRCA consists of a bone marrow aspirate
examination and assessment for the presence of anti-EPO
antibodies. Bone marrow aspirate reveals severe erythroid
hypoplasia, with <5% red blood cell precursors. Evidence of
maturation block of erythroid precursors may be present.
Platelet and white cell precursors are usually entirely normal.
Identification of anti-EPO antibodies are critical component of
the diagnosis. Several tests are available in special laboratories
including.
F. Radioimmunoprecipitation Assay (RIPA), which is the
most accurate test for detecting anti-EPO antibodies. It is time
consuming and difficult to automate [11].
G. Enzyme Linked Immunosorbent Assay (ELISA) are widely
available but have lower sensitivity and specificity than RIPA
[11,26]
H. A biosensor assay, not readily available, but may provide
better detection of antibodies.
I. Both neutralizing and non-neutralizing anti-EPO
antibodies may be present and can cause the PRCA. The
availability of these tests differs in different parts of the world. It
is recommended if you suspected the diagnosis of PRCA to send
the blood samples to the ESA-manufacture, where different test
can be performed [27].
J. There is limited experience with managing PRCA, but the
following have been used
K. Transfusions for symptomatic anemia and discontinuation
of all EPO products.
L. Immunosuppressive therapy to eradicate antibodies
in the form of corticosteroids alone or in combination with
cyclophosphamide, Intra-Venous Immune Globulin (IVIG),
plasmapheresis.
M. Cyclosporin or tacrolimus alone or in combination with
steroids.
N. Mycophenolate mofetil plus or minus rituximab
O. Kidney transplant
Patients who received cyclophosphamide or cyclosporin with or without steroids have the best results. The fastest response occurred with the use of cyclosporin [28-31]. Continuation of treatments with corticosteroids and immunotherapy should be accomplished until the anti-EPO antibodies become undetectable. The treatment can be discontinued in patients who do not respond in 3-4 months after initiation of therapy. Monitoring reticulocyte count and anti-EPO antibody levels every 1-2 weeks during treatment is recommended. Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) are a novel class of oral drugs that stimulate the production of endogenous EPO. These drugs can be helpful for the management of anemia in patients with EPOassociate PRCA [32-35].
Conclusion
Patients who develop normocytic normochromic anemia while on ESA with normal WBC, platelet count, and normal iron stores should be investigated for bone marrow failure or PRCA. PRCA is characterized by a normocytic normochromic anemia with severe reticulocytopenia and marked reduction in the erythroid precursors in the bone marrow. The white blood cell and the platelet counts are usually normal. Most cases of EPO-related PRCA are due to anti-EPO antibodies. Treatment consists of discontinuation of ESA, transfusion of RBCs as necessary for anemia, immunosuppression to eradicate the antibodies, and at times kidney transplant in patients who are transplant candidates.
References
- Fisch P, Handgretinger R, Schaefer HE (2000) Pure red cell aplasia. Br J Haematol 111(4): 1010-1022.
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, et al. (2002) Pure red-cell aplasia and anti-erythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med 346(7): 469-475.
- Krantz S (1980) Anemia due to bone marrow failure: Diagnosis and treatment. Compr Ther 6(7): 10-19.
- Casadevall N, Dupuy E, Molho-Sabatir P, Tobelem G, Varet B, et al. (1996) Autoantibodies against erythropoietin in a patient with pure red cell aplasia. N Engl J Med 334(10): 630-633.
- Bunn HF (2002) Drug-induced autoimmune red-cell aplasia. N Engl J Med 346(7): 522-523.
- Peschle C, Marmont AM, Marone G, Genovese A, Sasso GF, et al. (1975) Pure red cell aplasia: Studies on an IgG serum inhibitor neutralizing erythropoietin. Br J Haematol 30(4): 411-417.
- Storring PL, Tiplady RJ, Gaines Das RE, Stenning BE, Lamikanra A, et al. (1998) Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol 100(1): 79-89.
- Padhi S, Behera G, Pattnaik SA, Das PK, Adhya AK, et al. (2020) Acquired pure red cell aplasia following recombinant erythropoietin (Darbepoetin-alfa) therapy. Indian J Nephrol 30(2): 113-116.
- Shingu Y, Nakata T, Sawai S, Tanaka H, Asai O, et al. (2020) Antibody-mediated pure red cell aplasia related with epoetin-beta pegol (C.E.R.A) as an erythropoietic agent: case report of a dialysis patient. BMC Nephrol 21(1):275.
- Quint L, Casadevall N, Giraudier S (2004) Pure red cell aplasia in patients with refractory anaemia treated with two different recombinant erythropoietins. Br J Haematol 124(6): 842.
- Rossert J, Casadevall N, Eckardt KU (2004) Anti-erythropoietin antibodies and pure red cell aplasia. J Am Soc Nephrol 15(2): 398-406.
- Eckardt KU, Casadevall N (2003) Pure red-cell aplasia due to anti-erythropoietin antibodies. Nephrol Dial Transplant 18: 398
- Bennett CL, Luminart S, Nissenson AR, Tallman MS, Klinge SA, et al. (2004) Pure red-cell aplasia and epoetin therapy. N Engl J Med 351(14):1403-1408.
- Gershon SK, Luksenburg H, Vote TR, Braun MM (2002) Pure red-cell aplasia and recombinant erythropoietin. N Engl J Med 346(20): 1584-1586.
- Peces R, de la Torre M, Alcazar R, Urra JM (1996) Antibodies against recombinant human erythropoietin in a patient with erythropoietin-resistant anemia. N Engl J Med 335(7): 523-524.
- Boven K, Stryker S, Knight J, Thomas A, Regenmortel MV, et al. (2005) The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int 67(6): 2346-2353.
- Macdougall IC (2005) Antibody-mediated pure red cell aplasia (PRCA): epidemiology, immunogenicity and risks. Nephrol Dial Transplant 20 Suppl 4: iv9-15.
- McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, et al. (2008) Epoetin-associated pure red cell aplasia: Past, present, and future consideration. Transfusion 48(8): 1754-1762.
- Haag-Weber M, Eckardt KU, Horl WH, Roger SD, Vetter A, et al. (2012) Safety, immunogenicity and efficacy of subcutaneous biosimilar epoetin-a (HX575) in non-dialysis patients with renal anemia: A muti-center, randomized, double-blind study. Clin Nephrol 77(1): 8-17.
- Praditpornsilpa K, Tiranathanagul K, Kupatawintu P, Jootar S, Intragumtornchai T, et al. (2011) Biosimilar recombinant human erythropoietin induces the production of neutralizing antibodies. Kidney Int 80(1): 88-92.
- Panichi V, Ricchiuti G, Scatena A, Vecchio LD, Locatelli F, et al. (2016) Pure red cell aplasia induced by epoetin zeta. Clin Kidney J 9(4): 599-602.
- Fishbane S, Singh B, Kumbhat S, Wisemandle WA, Martin NE, et al. (2018) Intravenous epoetin alfa-epbx versus epoetin alfa for treatment of anemia in end-stage kidney disease. Clin J Am Soc Nephrol 13(8): 1204-1214.
- Stoffel MP, Haverkamp H, Kromminga A, Lauterbach KW, Baldamus CA, et al. (2007) Prevalence of anti-erythropoietin antibodies in hemodialysis patients without clinical signs of pure red cell aplasia. Comparison between hypo- and normo-responsive patients treated with epoetins for renal anemia. Nephron Clin Pract 105(2): c90-c98.
- Wu G, Wadgymar A, Wong G, Ting R, Nathoo B, et al. (2004) A cross-sectional immunoveillance study of anti-EPO antibody levels in CRF patients receiving epoetin alfa in 5 ontario renal centers. Am J Kidney Dis 44(2): 264-269.
- (2012) Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int Suppl 2(4): 288-291.
- Swanson SJ, Ferbas J, Mayeux P, Casadevall N (2004) Evaluation of methods to detect and characterize antibodies against recombinant human erythropoietin. Nephron Clin Pract 96(3): c88-95.
- (2005) Amgen inc, medical information. Direct communication.
- Verhelst D, Rossert J, Casadevall N, Krüger A, Eckardt KU, et al. (2004) Treatment of erythropoietin-induced pure red cell aplasia: A retrospective study. Lancet 363(9423): 1768-1771.
- Chng WJ, Tan LK, Liu TC (2003) Cyclosporine treatment for patients with CRF who developed pure red blood cell aplasia following EPO therapy. Am J Kidney Dis 41(3): 692-695.
- Hashimoto K, Harada M, Kamijo Y (2016) Pure red cell aplasia induced by anti-erythropoietin antibodies, well-controlled with tacrolimus. Int J Hematol 104(4): 502-505.
- Bennett CL, Cournoyer D, Carson KR, Rossert J, Luminari S, et al. (2005) Long-term outcome of individuals with pure red cell aplasia and anti-erythropoietin antibodies in patients treated with recombinant epoetin: A follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project. Blood 106(10): 3343-3347.
- Zhang H, Huang Z, He L, Yuan F, Sun L, et al. (2020) Successful treatment of anti-EPO antibody associated refractory anemia with hypoxia-inducible factor prolyl hydroxylase inhibitor. Ren Fail 42(1): 860-864.
- Wan K, Yin Y, Luo Z, Cheng J (2021) Remarkable response to roxadustat in a case of anti-erythropoietin antibody-mediated pure red cell aplasia. Ann Hematol 100(2): 591-593.
- Cai KD, Zhu BX, Lin HX, Luo Q (2021) Successful application of roxadustat in the treatment of patients with anti-erythropoietin antibody-mediated renal anaemia: A case report and literature review. J Int Med Res 49(4): 30000605211005984.
- Wu R, Peng Y (2021) Roxadustat on anti-erythropoietin antibody-related pure red cell aplasia in patient with end-stage renal disease. Semin Dial 34(4): 319-322.
© 2023 Magbri A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






