- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
CMR Perfusion with Quantitative Analysis and the Changing Role of Myocardial Ischemia
Guillem Pons-Lladó
Head (Emeritus) Cardiac Imaging Unit Cardiology Department Hospital de Sant Pau Universitat Autònoma de Barcelona Clínica Creu Blanca Barcelona, Spain
*Corresponding author: Guillem Pons- Lladó, Head (Emeritus) Cardiac Imaging Unit Cardiology Department Hospital de Sant Pau Universitat Autònoma de Barcelona Clínica Creu Blanca Barcelona, Spain
Submission: November 01, 2022;Published: November 23, 2022

ISSN 2578-0204Volume4 Issue1
Opinion
The role of myocardial ischemia in patients with stable coronary artery disease has been questioned after several well-done, large trials found no statistical difference in cardiovascular events with an initial invasive strategy as compared with a conservative one [1,2], even in the long run [3]. These studies have been carefully scrutinized [4,5] as, in clinical practice, it seems odd that the relief of the ischemic burden of the heart may have a negligible impact on an illness called “Ischemic” Heart Disease (IHD). On the other hand, the proven beneficial effect of intervention after a rigorous preselection of patients with truly flow-limiting coronary lesions evidenced by physiological invasive measures [6,7] contrasts with this notion, suggesting a rationale for choosing the right target at the time of vessel revascularization. This has opened interesting debates on several aspects of the issue. One of them, of special relevance in our opinion, is the study of the presence and grading of myocardial ischemia performed in these trials. Forcibly, it was based on the usual diagnostic techniques available, i.e.: stress EKG, stress echocardiography, nuclear perfusion imaging, and, in the most recently studied patients, also Cardiovascular Magnetic Resonance (CMR) contrast first-pass perfusion.
Although all of these methods were appropriately analyzed in a core laboratory [5], a common feature of them all is their subjective evaluation, where inducible ischemia is judged to be present when EKG changes, regional contractile abnormalities, or perfusion defects, appeared under stress conditions, seem quite evident to the operator. The issue of stratifying the degree of ischemia, though predefined by consensus [8] and adjusted to current clinical standards, is also elementary, as is merely based on the extension of defects, again depending on the judgment of the observer. Whatever it may be -if any- the confounding potential of these limitations on the mentioned trials, there seems to be room for improvement in the assessment of myocardial ischemia for the appropriate management of patients with stable IHD [9]. This should involve a double aim: making it less dependent on subjective analysis and refining those criteria for a reliable grading.
The technique of CMR has been enriched recently with the availability of a reliable application of quantitative analysis of first-pass contrast myocardial perfusion studies developed by Kellman and associates [10].
Although quantification of CMR perfusion had been feasible long before [11], it remained largely confined to research purposes. This new tool, however, contains several outstanding solutions that allow the technique to become an optimal study method for the ischemic burden of the heart in current clinical practice. Based on a dual-sequence MR strategy, the Quantitative Perfusion Mapping (QPM) technique solves the challenging issue of deriving absolute values of Myocardial Blood Flow (MBF) from myocardial signal intensity changes through the time-lapse of the myocardial first pass of a contrast agent. Importantly, the process is done in-line automatically [12]. In addition, based on an extensive deep learning process [13], myocardial segmentation and allocation of flow values are automatically processed and integrated into the MR scanner using streaming reconstruction software [14]. The final output consists of a bull’s-eye plot of color-encoded MBF values on the myocardial 16-segment model, and the listed numerical values of absolute rest and stress flow (in ml/min/g) and, also, the derived Myocardial Perfusion Reserve (MPR), each of them calculated for the endo- and epicardial halves of every segment.
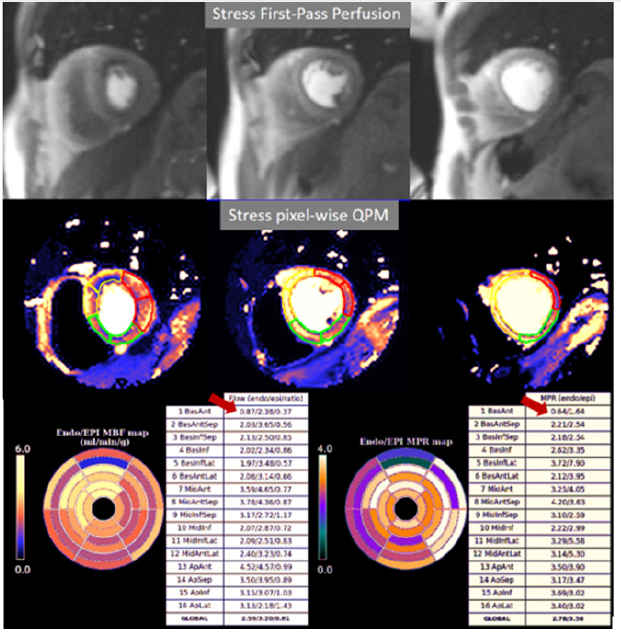
The presence of inducible perfusion defects is thus detected not only visually on the raw perfusion images and color map, but also quantitatively estimated by the absolute values of stress MBF and the corresponding MPR of the involved myocardial segments (Figure 1). A most remarkable feature of the dual sequence QPM is the fully automated image processing and reconstruction which is performed in-line without any user interaction and displayed on the scanner within minutes, which contributes to its feasibility and reproducibility [15]. Despite its recent introduction, dualsequence QPM has been exhaustively validated, with excellent results, in comparison studies with Positron Emission Tomography [16], still considered as the reference in myocardial blood flow quantitation, although scarcely available in routine clinical practice due to logistical reasons. Also, QPM has been successfully compared with coronary angiography, either quantitative [17], or assisted by invasive measures of coronary physiology [18]. These studies allowed for the definition of thresholds of Stress MBF to detect significant epicardial coronary artery lesions and coronary Microvascular Dysfunction (MVD).
Figure 1:Top row: Images from a CMR perfusion study at maximal stress where a subendocardial defect appears to be present in most of the segments, leading to the suspicion of moderate-to-severe ischemia. Middle row: Corresponding pixel-wise maps of quantitative myocardial perfusion where darker colors identify areas with relatively lower values of MBF. Bottom row: Panels of endo/epicardial absolute values of Stress MBF (left) and MPR (right) showing reduced values of both parameters only in the subendocardial region of the basal anterior segment (arrows), indicating a mild degree of ischemia. Rest perfusion images and quantitative maps are not shown due to space constraints.
Essential for the consideration of QPM as a clinically useful tool are those studies of prognostic impact. To date, a large study (>1,000 patients with a mean follow-up of >1.5 years) has been published [19] showing that stress MBF and MPR are strong, independent predictors of adverse cardiovascular outcomes. Further reports on longitudinal studies, now on their way, will add more information enlarging these data, and allowing QPM to be consolidated in practice as a unique tool in the arsenal of CMR resources [20]. This new body of information from CMR myocardial perfusion has not only the potential for detecting conditions poorly known such as MVD but also for refining the prognostic stratification of patients with stable IHD, making “significant” inducible ischemia a reliable concept that, in consequence, should imply a beneficial effect from revascularization. The consideration of myocardial ischemia in stable IHD is thus still open to new insights, and QPM seems to be equipped to play a primary role in this field.
Conflict of Interest
None to declare.
References
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, et al. (2007) Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356(15): 1503-1516.
- Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, et al. (2020) Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 382(15): 1395-1407.
- Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, et al. (2015) Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 373 (20): 1937-1946.
- Gibbons RJ (2021) Myocardial ischemia in the management of chronic coronary artery disease: Past and present. Circ Cardiovasc Imaging 14 (1): e011615.
- Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, et al. (2021) Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation 144(13): 1024-1038.
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, et al. (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367(11): 991-1001.
- Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, et al. (2018) Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 379(3): 250-259.
- Shaw LJ, Berman DS, Picard MH, Friedrich MG, Kwong RY, et al. (2014) Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging 7(6): 593-604.
- Adusumalli S, Giri J (2019) Paradigm shifts in the treatment of stable ischemic heart disease. Where do we draw the line? Circ Cardiovasc Qual Outcomes 12(2): e005388.
- Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, et al. (2017) Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 19(1): 43.
- Jerosch-Herold M, Wilke N, Stillman AE (1998) Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys 25(1): 73-84.
- Xue H, Brown LAE, Nielles-Vallespin S, Plein S, Kellman P (2020) Automatic in-line quantitative myocardial perfusion mapping: Processing algorithm and implementation. Magn Reson Med 83(2): 712-730.
- Xue H, Tseng E, Knott KD, Kotecha T, Brown L, et al. (2020) Automated detection of left ventricle in arterial input function images for inline perfusion mapping using deep learning: A study of 15,000 patients. Magn Reson Med 84(5): 2788-2800.
- Xue H, Inati S, Sørensen TS, Kellman P, Hansen MS (2015) Distributed MRI reconstruction using gadgetron-based cloud computing. Magn Reson Med 73(3): 1015-1025.
- Brown LAE, Onciul SC, Broadbent DA, Johnson K, Fent GJ, et al. (2018) Fully automated, inline quantification of myocardial blood flow with cardiovascular magnetic resonance: Repeatability of measurements in healthy subjects. J Cardiovasc Magn Reson 20(1): 48.
- Engblom H, Xue H, Akil S, Carlsson M, Hindorf C, et al. (2017) Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: a comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reason 19(1): 78-87.
- Hsu LY, Jacobs M, Benovoy M, Ta AD, Conn HM, et al. (2018) Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 11(5): 697-707.
- Kotecha T, Martínez-Naharro A, Boldrini M, Knight D, Hawkins P, et al. (2019) Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging 12(10): 1958-1969.
- Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, et al. (2020) The prognostic significance of quantitative myocardial perfusion: an Artificial Intelligence-based approach using perfusion mapping. Circulation 141(16): 1282-1291.
- Pons-Lladó G, Kellman P (2022) State-of-the-Art of myocardial perfusion by CMR: A practical view. Rev Cardiovasc Med 23(10): 325-344.
© 2022 Pons-Lladó G. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






