- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Varicella Zoster Virus and Cardiovascular Diseases: The Opinion of the Cardiologist
Angelica C, Mauro R, Ludovica A, Giuliana C, Gianmarco A, Marco M and Enrico V*
Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Italy
*Corresponding author: Enrico V, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Italy
Submission: July 27, 2022;Published: October 14, 2022

ISSN 2578-0204Volume3 Issue5
Abstract
Varicella Zoster Virus (VZV) is a double-stranded DNA virus from the Herpesviridae family that affects humans only. The first clinical manifestation appears to be varicella, typical of childhood. After primary infection, however, VZV becomes latent in ganglion neurons along the entire neuroaxis. In the elderly and in immunocompromised individuals, the VZV reactivates and travels along the peripheral nerve fibers resulting in Zoster. However, it can also spread centrally and infect cerebral and extracranial arteries resulting in vasculopathy that can result in transient ischemic attacks, strokes, aneurysms, cavernous sinus thrombosis and giant cell arteritis, as well as granulomatous aortitis. The mechanisms of virusinduced pathological vascular remodeling are not fully understood; however, recent studies suggest that the inflammation and dysregulation of ligand-1 programmed death play a significant role. Few studies, on the other hand, evaluate the involvement of VZV in cardiovascular disease. This review therefore aims to analyze the link between VZV and cardiovascular disease, the efficacy of the vaccine as a protective mechanism and the target population of heart disease patients who could benefit from vaccination.
Keywords: VZV; HF; ACS; Vaccination
Introduction
Varicella Zoster Virus (VZV) is a human-only neurotropic alpha-herpesvirus that infects more than 95% of the world’s population. Primary infection typically results in varicella, followed by the establishment of latency of the virus in neurons of the cranial nerves, dorsal root and autonomic ganglia along the entire neuroaxis, as well as the adrenal glands. With a decline in VZV-specific cell-mediated immunity in elderly and immunocompromised individuals, defects in innate immunity (especially Natural Killer cell defects), or the presence of anti-cytokine antibodies, the virus reactivates by one or more ganglia, it travels peripherally from nerve fibers to the skin and produces herpes zoster in the corresponding dermatomes. The secondary manifestation (Zoster) is often complicated by post-herpetic neuralgia [1-7].
Impact of VZV on cardiovascular disease
VZV infections were first discovered to be associated with VZV neurological vasculopathy in 1919, when it was described as a late contralateral hemiplegia following a stroke [8]. Since then, there have been numerous reports of heart attacks in the brain, cerebellum, midbrain following VZV infection [9]. The mechanism of vasculopathy was initially proposed as a varicewlla infection leading to granulomatous angiitis [10,11]. Recently, VZV has been identified as a risk factor for stroke, but there have been few studies on the relationship between VZV and other cardiovascular diseases, including Myocardial Infarction (MI) or Heart Failure (HF) [12]. Yang et al. [13], evaluating all relevant studies and comparing them in a meta-analysis, demonstrated that VZV is a cause of cardiovascular events, probably due to the migration of VZV from neurons to the cerebral and coronary vascular system. This can lead to a local inflammatory response, causing occlusion of the vessels and ultimately ischemia.
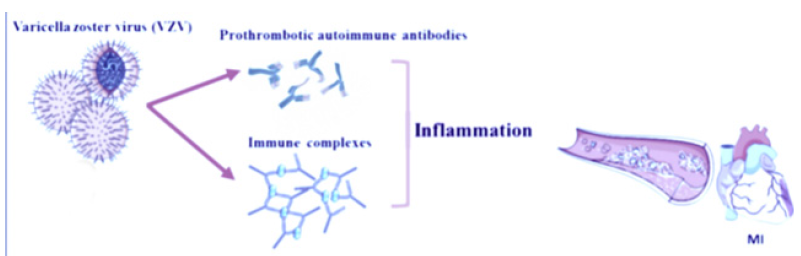
In fact, herpes zoster infection can induce ischemia through different mechanisms: firstly, through the production of various prothrombotic autoimmune antibodies such as IgM and IgG anticardiolipin and lupus anticoagulant [14]. On the other hand, IgM and IgG antiphospholipid antibodies have been reported in two patients with acute thrombosis of the deep femoral and tibial arteries following varicella pneumonia [15]. Secondly, as a result of VZV infection, delayed vasculopathy can develop due to an autoimmune phenomenon induced by circulating immune complexes [16] (Figure 1). Finally, direct diffusion of the transaxonal VZV from the dorsal root ganglia can cause vasculopathy and subsequent ischemia characterized by rupture of the internal elastic lamina, intimal hyperplasia and decreased smooth muscle cells in the medial layer [17]. More recently, Seo et al. [18] demonstrated that VZV patients requiring hospitalization are at risk for cardiovascular disease (CVD) including Myocardial Infarction (MI), ischemic stroke and Heart Failure (HF), and that patients with newly diagnosed CVD are also at risk of serious manifestations of VZV infection. Therefore, greater attention may be warranted in treating patients with VZV and concomitant CVD early [19-21].
Figure 1:Mechanisms of cardiovascular events associated with VZV infection.
Effects of the vaccine on cardiovascular disease
In 2006, a vaccine was approved to prevent VZV in immunocompetent elderly [22]. The VZV vaccine, originally developed and licensed as a “varicella vaccine”, is a live attenuated vaccine effective in preventing primary infection with wildtype VZV. However, initial studies suggested that a higher titer of live attenuated virus would be required to elicit a significant and sustained increase in cell-mediated immunity in the elderly, possibly due to the elderly’s reduced responsiveness to vaccination in general. Consequently, on May 25, 2006, the Food and Drug Administration cleared the zoster vaccine for the prevention of Herpes Zoster in people aged 60 and over. The new commercially available vaccine VZV (Zostavax, Merck) specific for protection against shingles contains a minimum of 19,400 plaque-forming units per dose [23]. The preventive effect of live zoster vaccine Zostavax is thought to be a consequence of its potentiating effect on an elderly person’s cell-mediated immunity to VZV, mimicking the immunological benefits of chickenpox exposure of an adult immune to VZV. This pharmacological push pushes cell-mediated immunity to a new set point above the “immunological threshold” below which a person is at risk for Zoster [24].
As for side effects, within the first 42 days after vaccination, varicella-like rashes at the injection site are more frequent in recipients of the vaccine. Other symptoms and signs at the injection site that have occurred more frequently include erythema, localized pain or tenderness, swelling and itching [22,25]. The reduced efficacy of the Zostavax vaccine in particular in elderly individuals which reaches 37.6% in over 70s and 18% in over 80s together with the contraindication of use in most immunocompromised individuals as a live attenuated vaccine imposed the need for use a vaccine with different characteristics both in terms of type and desirable efficacy, taking into account the problems of using the live attenuated vaccine (ZVL). The new RZV vaccine (Shingrix, Gsk) indicated for the prevention of HZ and post herpetic neuralgia is an inactivated adjuvanted recombinant vaccine. It was authorized in the USA in 2017 by the FDA and in Europe it obtained the latest authorization concerning the extension of use for IC subjects in 2020.
It is currently available in Italy with indication from the age of 50 for immunocompetent subjects and from the age of 18 for IC subjects. Shingrix is a non-live subunit vaccine composed of an antigen, the surface structural glycoprotein E of the VZV and an adjuvant system AS01B designed to overcome the decline in cell-mediated immunity associated with immuno senescence, these aspects play a fundamental role in guaranteeing the longterm efficacy and the possibility of use in fragile populations by providing a broad overall protection to all the subjects for which it is indicated. The efficacy of the RZV vaccine exceeding 91.4% in the over 80 population was evaluated in the phase III clinical studies ZOE-50 and ZOE-70, conducted on over 30,000 participants, the two-dose schedule demonstrated a protection against HZ in all subjects over 50 with no safety concerns reported. Several studies have been carried out to evaluate the efficacy of the RZV vaccine in patients with increased risk and these populations involved IC subjects belonging to various types such as: subjects with HIV, transplanted (HSCT), onco-haematological patients, with solid tumors and with kidney transplants [26,27].
Discussion
The incidence rate of Acute Coronary Syndrome (ACS) is significantly higher among older patients than among younger patients. This implies that reactivation of VZV infection among older people is found to be associated with more severe complications with a higher hospitalization rate per case and a longer average hospital stay. Therefore, reactivation of VZV infection among older people may contribute to greater complications and worse prognosis [28]. Antiviral treatment of herpes zoster infection successfully reduced the cumulative risk of ACS. Indeed, patients who received inpatient antiviral treatments had higher underlying comorbidities and a higher intrinsic risk of developing cardiovascular disease than patients who did not receive antiviral treatment. In addition, in patients requiring inpatient antiviral therapy, there was a higher incidence of comorbidities such as hypertension, diabetes mellitus, dyslipidemia and cerebrovascular events. These risk factors are responsible for the impairment of cellular immunity and this entails both an increased risk of reactivation of VZV with consequent severe manifestations of the infection itself, and an increased risk of cardiovascular disease [20,29].
Heymann et al. [30] found, in fact, that individuals diagnosed with diabetes mellitus had a significantly higher risk of herpes zoster infection, regardless of age. Regarding the vaccine, Oxman et al. [31] demonstrated that the vaccine reduces the risk of developing VZV reinfection by 51.3% in individuals aged 60 years and that it is 66.5% effective in preventing Postherpetic neuralgia in this age group, improving both humoral immune responses and the risk of cardiovascular disease due to severe clinical manifestations of VZV [32-34]. The vaccine boosts VZV-specific immunity even in those patients with cardiovascular risk factors, especially those with diabetes mellitus. Additionally, the vaccine has been shown to be more effective at preventing shingles among people aged 60 to 69 than in those aged 70 and over [22]. In an American cohort study by Yang Q et al. [35], administration of the Zostavax vaccine was associated with a reduction in the risk of stroke compared to unvaccinated, during a median follow-up of 5 years, of approximately 16% [35].
VZV vaccination is therefore recommended in patients with cardiovascular diseases who have symptoms such as fatigue, dyspnoea, angor or heartbeat during less than ordinary physical activity (NYHA III) or symptoms at rest (NYHA IV). However, there is no scientific evidence that vaccination cannot be recommended even in patients with symptoms during ordinary physical activity (NYHA II) or no symptoms during ordinary physical activity (NYHA I) [36,37]. In a cohort study, heart failure patients had a 2.07-fold increased risk, compared to the general population, of developing herpes zoster infection at a one-year follow-up. The responsible mechanism remains unclear even though the dysfunction of natural killer cells, found in patients with heart failure, may be related to reactivation [38]. For this reason, it can be assumed that vaccination for Herpes Zoster is useful in patients with heart failure to prevent herpetic over-infection with consequent risk of major cardiovascular complications and hospitalization. However, it must be taken into account that chronic heart failure induces an immense expansion of T cells, which contributes to an overall reduction in the pool of naïve T cells and, therefore, a greater degree of immunosenescence. This would explain the observed reduction in vaccine immunogenicity [39].
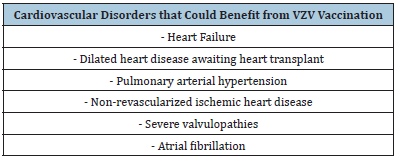
Data in the literature also showed a correlation between viral infections, including human herpesvirus-8, with angioproliferation and the development of pulmonary arterial hypertension in genetically predisposed subjects [40]. For this reason, we can hypothesize a role in pulmonary hypertension also of other viruses of the Herspesviridae family including VZV. This data may support the use of VZV vaccination in patients with pulmonary arterial hypertension in order to avoid aggravation of the symptoms. Furthermore, the intrinsic cardiac autonomic nervous system plays a critical role in the initiation and maintenance of Atrial Fibrillation (AF), and sympathetic-vagal discharges are common triggers for paroxysmal AF. Herpes Zoster can involve the nerve ganglia, resulting in autonomic dysfunction. Consequently, an increased risk of atrial fibrillation has also been found in patients with Herpes Zoster, especially in the severe forms [41]. Consequently, given that patients with severe forms of VZV are at risk of CVD, including myocardial infarction and heart failure, and that patients with CVD are at risk for severe manifestations of VZV infection, the vaccination offer could be expanded to a large population of cardiopathic patients (Table 1), regardless of the presence of class NYHA> 3. The impact of VZV vaccination on the healthy population in primary prevention of cardiovascular diseases still has to be evaluated.
Table 1:Cardiovascular disorders that could benefit from VZV vaccination.
Conclusion
Varicella Zoster virus is responsible for the development of cardiovascular diseases such as heart failure, ischemic stroke or myocardial infarction. In addition, patients with cardiovascular disease are at greater risk of severe forms of the Varicella Zoster virus. For this reason, vaccination is offered on active call in patients with cardiovascular diseases. Further clinical studies are needed to define with certainty which cardiovascular diseases would benefit most from this vaccination and the impact of varicella zoster vaccination in the healthy population in primary prevention on cardiovascular diseases.
Conflict of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding
The authors report no funding.
References
- Badani H, White T, Schulick N, Raeburn CD, Topkaya I, et al. (2016) Frequency of varicella zoster virus DNA in human adrenal glands. J Neurovirol 22(3): 400-402.
- Baudouin E, Lantuejoul P (1919) Motor disorders in shingles. Hospital Gazette.
- Braun KP, Bulder MM, Chabrier S, Kirkham FJ, Uiterwaal CS, et al. (2009) The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 132(Pt 2): 544-557.
- Breuer J, Pacou M, Gauthier A, Brown MM (2014) Herpes zoster as a risk factor for stroke and TIA: A retrospective cohort study in the UK. Neurology 82(3): 206-212.
- Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, et al. (2010) Anti- cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 116(23): 4848-4858.
- Nagel MA, Bubak AN (2018) Varicella zoster virus vasculopathy. J Infect Dis 218(suppl_2): S107-S112.
- Nagel MA, Jones D, Wyborny A (2017) Varicella zoster virus vasculopathy: The expanding clinical spectrum and pathogenesis. J Neuroimmunol 308: 112-117.
- Cope S, Jones AT (1954) Hemiplegia complicating ophthalmic zoster. Lancet 267(6844): 898-899.
- Hashemi N, Zhang J, Volpi J, Lee AG, Gordon LK (2013) A pox upon your house. Surv Ophthal- mol 58(6): 640-643.
- Filloux F, Townsend J (1985) Herpes zoster ophthalmicus with ipsilateral cerebellar infarction. Neurology 35(10): 1531-1532.
- Verghese A, Sugar AM (1986) Herpes zoster ophthalmicus and granulomatous angiitis. An ill-appreciated cause of stroke. J Am Geriatr Soc 34(4): 309-312.
- Liu X, Guan Y, Hou L, Huang H, Liu H, et al. (2016) The short- and long-term risk of stroke after herpes zoster: a meta-analysis. PLoS ONE 11(10): e0165203.
- Yang SY, Li HX, Yi XH, Han GL, Zong Q, et al. (2017) Risk of stroke in patients with herpes zoster: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis 26(2): 301-307.
- Uthman I, Taher A, Khalil I (2001) Hughes syndrome associated with varicella infection. Rheumatol Int 20(4): 167-168.
- Peyton BD, Cutler BS, Stewart FM (1998) Spontaneous tibial artery thrombosis associated with varicella pneumonia and free protein S deficiency. J Vasc Surg 27(3): 563-567.
- Bodensteiner JB, Hille MR, Riggs JE (1992) Clinical features of vascular thrombosis following varicella. Am J Dis Child 146(1): 100-102.
- Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, et al. (2017) A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One 12(7): e0181565.
- Seo HM, Cha MJ, Han JH, Han K, Park SH, et al. (2018) Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in Korea. J Dermatol. 2018 Nov;45(11): 1312-1318.
- Warren-Gash C (2018) Herpes Zoster: Epidemiological Links With Stroke and Myocardial Infarction. J Infect Dis 218(suppl_2): S102-S106.
- Wang CC, Lin CL, Chang YJ, Wang GJ, Sung FC, et al. (2014) Herpes zoster infection associated with acute coronary syndrome: a population-based retrospective cohort study. Br J Dermatol 170(5): 1122-1129.
- Wu PH, Chuang YS, Lin YT (2019) Does herpes zooster increase the risk of stroke and myocardial infarction? A comprehensive review. J Clin Med 8(4): p. 547.
- Kimberlin DW, Whitley RJ. Varicella-zoster vaccine for the prevention of herpes zoster. N Engl J Med. 2007;356(13):1338-1343.
- Zostavax (2006) Whitehouse Station, NJ: Merck & Co, USA.
- Levin MJ, Murray M, Rotbart HA, Zerbe GO, White CJ, et al. (1992) Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis 166(2): 253-259.
- Schmader KE, Levin MJ, Gnann JW, McNeil SA, Vesikari T, et al. (2012) Efficacy, Safety and Tolerability of Herpes Zoster Vaccine in Persons aged 50-59 years. Clin Infect Dis 54(7): 922-928.
- Shingrix, Scientific Dossier
- Shingrix, Summary of product characteristics
- Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, et al. (2001) Epidemiology of varicella zoster infection in Canada and the United Kingdom. Epidemiol Infect 127(2):305-314.
- Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y, et al. (2009) Comparison of varicella zoster virus specific immunity to patients with diabetes mellitus and healthy individuals. J Infect Dis 200(10): 1606-1610.
- Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, et al. Diabetes as a risk factor for herpes zoster infection: Results of a population-based study in Israel. Infection 36(3): 226-230.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352(22): 2271-2284.
- Arvin AM (1996) Varicella-zoster virus. Clin Microbiol Rev 9(3): 361-381.
- Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, et al. (2003) Decline in varicella-zoster virus (VZV)–specific cell-mediated immunity with increasing ageand boosting with a high-dose VZV vaccine. J Infect Dis 2003; 188(9): 1336-1344.
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, et al. (2008) Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 197(6): 825-835.
- Yang Q, Chang A, Tong X, Merritt R (2021) Herpes zoster vaccine live and risk of stroke among medicare beneficiaries. Stroke 52(5): 1712-1721.
- Ministry of Health, National Vaccine Prevention Plan 2017-2019.
- Indications for use of the anti-herpes zoster vaccine, circular from the Lombardy Region, Italy.
- Wu PH, Lin YT, Lin CY, Huang MY, Chang WC, et al. (2015) A nationwide population-based cohort study to identify the correlation between heart failure and the subsequent risk of herpes zoster. BMC Infect Dis 15: p. 17.
- Verschoor CP, Lelic A, Parsons R, Evelegh C, Bramson JL, et al. (2017) Serum C-Reactive Protein and Congestive Heart Failure as Significant Predictors of Herpes Zoster Vaccine Response in Elderly Nursing Home Residents. J Infect Dis 216(2): 191-197.
- Cool CD, Voelkel NF, Bull T (2011) Viral infection and pulmonary hypertension: is there an association? Expert Rev Respir Med 5(2): 207-216.
- Cha MJ, Seo HM, Choi EK, Lee JH, Han K, et al. (2018) Increased risk of atrial fibrillation in the early period after herpes zoster infection: A nationwide population-based case-control study. J Korean Med Sci 33(22): e160.
© 2022 Enrico V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






