- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Cardio Oncologist: A Prerequisite for Preventing Cardiac Damage in Cancer Therapy
Misbah Ul Haq M*1, Munaf Ur Razzak M1, Ahmed N1, Yousuf Younus M1 and Ahmed O2,3,4
1Deccan School of Pharmacy, Affiliated to Osmania University, India
2Rajiv Gandhi University of Health Sciences (RGUHS) - V.L College of Pharmacy, Pharmaceutical Chemistry, India
3Pacific (PAHER) University, Pharmaceutical Chemistry, India
4Acharya Nagarjuna University Centre for Distance Education, Human Resource Management, India
Misbah Ul Haq M, Deccan School of Pharmacy, Affiliated to Osmania University, India
Submission: February 14, 2022;Published: April 26, 2022

ISSN 2578-0204Volume3 Issue4
Abstract
To strengthen our understanding of cardiac oncology, we studied the fundamentals of oncology, focusing on the cardiovascular system and its effects on cardiotoxicity. The success of basic research and the study of specific diseases make cancer patients one of the fastest-growing subgroups. Interestingly, cardiovascular disorders are the 2nd major cause of illness and death after malignant tumors in cancer patients. A unique approach to addressing the early detection, prevention and treatment of cardiotoxicity in cancer patients has been proposed, for cancer patients as multidisciplinary cardiovascular care. Cancer therapies have been linked to a number of cardiovascular adverse effects, including arrhythmias, hypertension, and ischemic heart disease. This article highlights current practice guidelines for cancerrelated therapy’s potentially serious cardiotoxicity, as well as the importance, role, and need for a cardiooncologist.
Keywords: Cardiotoxicity; Cardio-Oncology; Cardiovascular system disease; Cardio-oncologist; Cancer
Introduction
Cardiotoxicity is a common adverse effect of chemotherapy. There have been earlier
reports of cardiac failure in children who had been given doxorubicin [1]. Since then,
anthracycline-based cytostatic antibiotics have been the most often utilized cardiotoxic
chemotherapeutic drugs. A number of other chemotherapy drugs can induce cardiotoxicity,
which many doctors are unaware of. Cardiomyopathy, arrhythmias, myocardial infarction,
myocarditis, and pericarditis, as well as mild transient blood pressure and ECG abnormalities,
can all cause CHF or LVD. The goal of this article is to discuss about the potential for serious
cardiotoxicity in current cancer therapies, as well as the following:
A. Cardiotoxicity caused by chemotherapy
B. Cancer and heart-induced cancer therapy
C. Common drug interactions between Cardiovascular Drugs
D. To show the importance of the role and need for a cardio-oncologist
Cardiotoxicity caused by chemotherapy
Chemotherapy’s basic principle is to impair the mitotic and metabolic processes of cancer cells. Unfortunately, chemotherapy affects certain normal cells and tissues, resulting in a variety of minor to severe side effects such as bone marrow suppression, nausea, and vomiting, as well as cardiovascular side effects such as hypotension, tachycardia, arrhythmias, and heart failure [1]. According to the National Cancer Institute, Cardiac Toxicity is defined as that affects the heart as cardiotoxicity. Cardiotoxicity is a broad term that should not simply encompass the difference in resting heart parameters. But also the cardiovascular system’s recruitable stroke work, coronary blood flow reserve, and dynamic functional evaluation of maximal functional capacity [VO2]. We know that many cancer survivors have decreased exercise capacity, which has a significant impact on their quality of life. Dynamic cardiovascular assessments are important because we know that many cancer survivors have decreased exercise capacity, which has a significant impact on their quality of life. Although various chemotherapeutic drugs can impact cardiotoxicity, as shown in (Table 1), certain kinds of chemotherapy, such as anthracyclines, cause more frequent and common adverse effects [2].
Table 1:Potential cardiotoxicity induced by numerous chemotherapeutic agents.
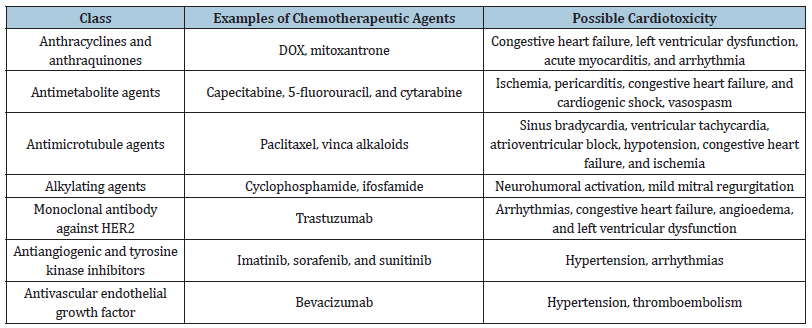
Patients at high risk of cardiac problems should not be given these medications. Cardiotoxicity is more likely in people with high blood pressure, diabetes, liver illness, or a history of heart disease [3]. Cardiotoxicity is also strongly influenced by the route of administration, the duration of chemotherapy (cumulative Dose), and the dose regimen. Combination of anthracyclines with other potentially cardiotoxic agents, such as paclitaxel or trastuzumab, would significantly enhance the risk of cardiotoxicity, potentially leading to severe heart failure [4-6]. It is even more critical for the health care practitioner to be aware of the cardiac side effects of cancer treatment. Although there is a lack of real consensus on definitions of cardiotoxicity, direct effects on cardiac structures (e.g., fibrosis), cardiac arrhythmias and conduction, systemic, diastolic, hemodynamic, hemostasis, thrombosis, and pulmonary vascular function, as well as a cardiac response to injury and stress, are all factors to consider [4]. During cancer therapy, the tone and specific cardiovascular biomarkers (e.g., troponin I and natriuretic peptides) may reflect subtle alterations of the cardiovascular system that are predictive for the development of HF before dropin LVEF [5-7].
Cancer and heart-induced cancer therapy
Chemotherapy commonly causes heart failure in cancer survivors, reducing their quality of life and increasing mortality. Classical chemotherapy, immunotherapy, radiation, surgery, and targeted therapies are all part of modern cancer treatment [8]. The most common cardiovascular complication associated with cancer therapy is heart failure. Both traditional and innovative cancer drugs contribute to the development of heart failure [9]. Cardiotoxicity is dose-dependent, but with lower cumulative doses it can also occur [10]. In patients with symptoms of heart failure, it is recommended to begin therapy with cardioprotective drugs. However, evidence-based guidance is limited, and the European cardio-oncology guidelines refer to heart failure recommendations that encourage the use of Angiotensin II Receptor Blockers (ARBs) / Angiotensin Converting Enzyme (ACE) inhibitors and beta-blockers [11-14]. Several studies have been conducted to evaluate whether such treatment, when administered prior to cancer treatment, might prevent the development of heart failure. There is currently limited data to variability in drug selection, trial populations, and cancer entities [15,16]. The most serious complication for cancer patients is still heart failure. Heart failure can develop during therapy or years after it has been completed. Alternative preventive medicines for younger patients (for example, breast cancer patients or children and adolescents with hematological malignancies) have not been thoroughly researched.
At the present, there are no indicators of guidelines. According to existing data, using ARB/ACE inhibitors or beta blockers for the management of heart failure before starting cancer therapy is associated with a moderate benefit in LVEF in some populations. Of course, patients should be warned about the potential side effects of these medications. The available studies differ with regard to the specific drug, the dosages and the timing (especially with regard to the timing of chemotherapy) and duration of use. Protective effects, which are observed, for example, with a certain beta block, cannot easily be demonstrated within the entire group of active substances (Nebivolol’s effect on nitric oxide metabolism). The efficacy of preventive treatments, the timing of drug administration, and monitoring should all be assessed in future research, given the clear correlation between cardiac dysfunction and these patients Furthermore, it remains to be seen whether the potential benefits of the prevention methods found in the studies studied still are evident after cancer treatment and the discontinuation of betablockers and ACE/ARB inhibitors, which would answer the question of whether beta-blockers and/or ACE are discontinued. Cardiotoxicity has been enhanced or specifically prevented by ACE inhibitors/ARBs. Surrogate outcomes such as left ventricular ejection fraction evaluated by Echocardiography (ECG) or magnetic resonance tomography (MRI), as well as clinical conditions, quality of life, and impermanence, were contradictory [17].
In canonical heart failure, beta blockers are used to decrease sympathetic activity, reduce heart rate, and improve excitationcontraction coupling. ACE/ARB inhibitors are medications that are used to reduce cardiac remodeling and control blood pressure. Finally, ACE inhibitors and beta-blockers ensure that LVEF is kept as low as possible [18]. These preventative treatments can be initiated for patients who are at risk of receiving standard highdose chemotherapy, especially those with risk factors. Pre-existing cardiovascular risk factors for chemotherapy-related heart failure, not only in terms of LVEF preservation, but also of quality of life and survival. It is also the immediate focus of cardio-oncology research, which is becoming increasingly important as new, more active chemotherapies become available.
Cardiotoxicity associated with radiation therapy
Radiation therapy is used to treat various types of cancer. Chest radiation can damage the pericardium, myocardium, valves, and coronary arteries, with the pericardium being the most commonly affected. Radiotherapy can cause silent vascular damage; roughly 50% of asymptomatic individuals acquire new myocardial perfusion defects. Most of the patients present with angina, dyspnea, or heart failure [19]. Despite the fact that sudden death has been reported [20]. Unexpected mortality in radiation therapy patients is thought to be caused by diffuse intimal hyperplasia of coronary arteries or left main stenosis [21]. It also causes pericardial fibrous thickening. Pericardial effusion is an early manifestation, while pericardial effusion is a late manifestation that generally arises after 18 months. Radiation treatment can potentially cause myocardial fibrosis. Fibrosis is characterized by significant changes in collagen synthesis [22]. Because radiation promotes fibrous thickening of the heart valves, valvular heart disease is also common following radiation [21,23].
Cardiovascular toxicity monitoring
Anticancer drugs related for monitoring cardiotoxic effects such as arrhythmias, ischemic cardiac events, and pericardial disease should be specifically planned and adapted to each therapeutic protocol under which anticancer agents are prescribed. Cardiac tests such as electrocardiography (ECG), myocardial perfusion a rest and stress imaging, and troponin levels to monitor ischemic cardiac complications. This ECG is crucial in detailed evaluation of valvular heart disease, evaluating pericardial disease and the left ventricular systolic and diastolic function. Doppler echocardiography can also be used to assess hemodynamic status, including involvement of pulmonary hypertension [24].
Drug interactions between chemotherapy and cardiovascular drugs in cancer patients with pre-existing cardiovascular disease
Figure 1:An overview of the cardiovascular side effects of chemotherapy and radiation [27]
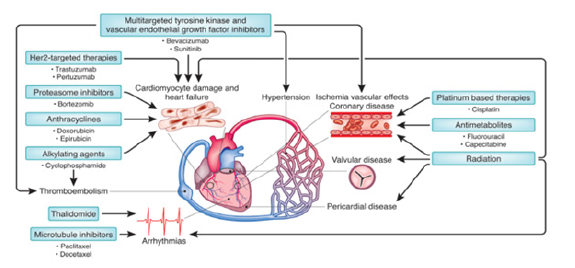
As the field of cardio oncology has advanced, the emphasis of treatment has switched from reactive to proactive therapy. Monitoring and short interruptions in cancer treatment are critical components of contemporary treatment paradigms.Heart medications such as beta-blockers, Angiotensin-Converting Enzyme Inhibitors (ACEI) or Angiotensin Receptor Blockers (ARBs), and statins are commonly used in combination with various chemotherapy drugs [25,26]. Because most chemotherapy treatments have a limited therapeutic window, factors that contribute to drug-drug interactions can improve or reduce chemotherapy efficacy or predispose patients to serious unfavorable side effects. These drug-drug interactions are very common in the cancer patients; one of the study showed that 16% patients had at least one serious interaction with drugs that could cause adverse side effects in patients receiving oral anticancer drugs. In the context of cancer treatment, healthcare practitioners should be mindful of possible drug-drug interactions and reduce complications when managing health of cardiovascular patients. In the following discussion of drug-drug interactions including common chemotherapy and cardiac drugs, Pharmacokinetics (PK) and Pharmacodynamic Interactions (PD), antithrombotic, and QT interval prolongation are all areas of concern (Figure 1).
Interactions Between Pharmacokinetics and Pharmacodynamics Mechanisms
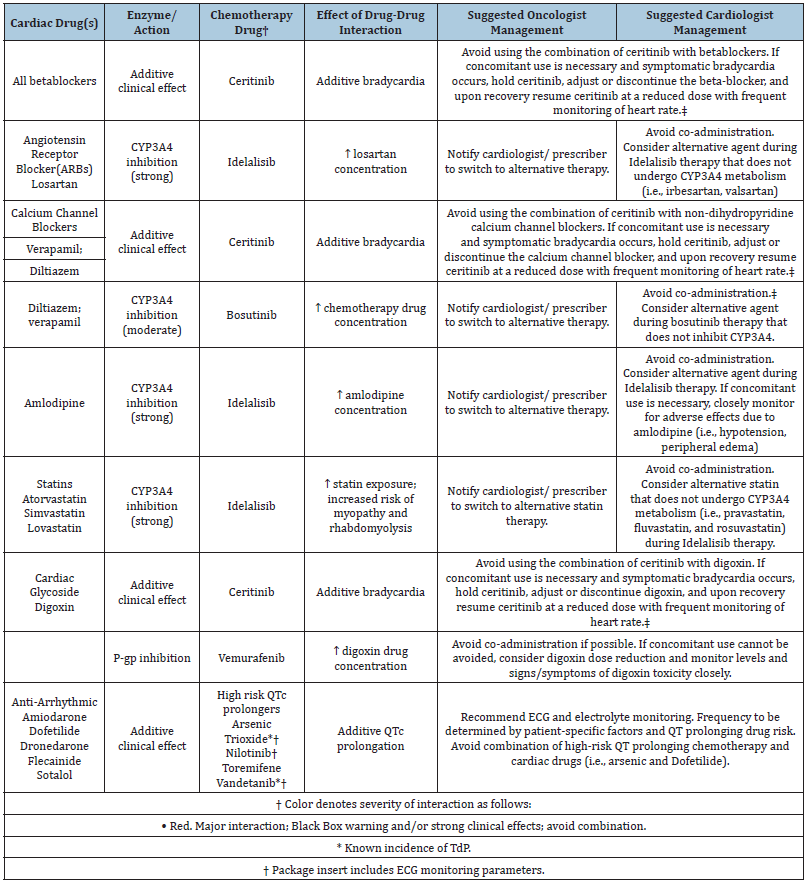
A pharmacodynamic and pharmacokinetic mechanism causes a drug-drug interaction. A pharmacodynamic interaction occurs when two drugs with identical molecular targets interact in a way that is additive or synergistic, resulting in an exaggerated clinical reaction or toxicity. The P-glycoprotein efflux pump and CYP450 enzymes are two of the most significant pharmacokinetic interactions in oncology [28,29]. The gastrointestinal tract metabolism is controlled by CYP enzymes [29]. Dose reduction or use of a different statin (different metabolic pathways). Atorvastatin, Simvastatin, and Lovastatin are all metabolized by CYP3A4. During Idelalisib therapy, we should consider other statins that do not undergo CYP3A4 metabolism (e.g., pravastatin, fluvastatin, and rosuvastatin) (as shown in table 2).
A variety of chemotherapeutic and cardiac drugs contain CYP450 inhibitors and substrates. They may be involved in dual drug interactions since they are both substrates and antagonists. P-glycoprotein (P-gp), an ATP binding cassette transporter family protein, is expressed in the intestinal epithelium and functions as an efflux pump, successfully exporting drugs from the cell cytoplasm to the lumen (gut); P-gp substrates include vinblastine, vincristine, doxorubicin and paclitaxel [29]. The concomitant administration of calcium channel blockers, beta blockers, and other medications causes polymorphic ventricular tachycardia. These medications may produce abnormally long QT intervals (Table 2).
Table 2:Drug-Drug interactions of common cardiac drugs and chemotherapeutic agents.
Cardio-Oncologist - Importance, Role and Need
The cardio-oncology association’s ultimate aim is to diagnose chemotherapy cardiotoxicity with a broad understanding, not to discourage or avoid oncological care, but to begin medical therapy or lifestyle modifications in the hopes of improving survival outcomes. In modern civilization, cancer and cardiovascular diseases are the leading causes of impermanence and death. Cured cancer patients aged 65 and over will increase from approximately 62% to 73% by 2040, which in turn will improve comorbidity [30]. The burden of cancer-related cardiovascular disease and cancer therapy was revealed in cancer patients with increasing age and survivors [31]. Early cardiotoxicity is observed in up to 48% of patients, and late cardiotoxicity can be found in 30% of patients 13 years after cancer treatment depending on the cancer treatment modalities [19,12,25]. Cardio-oncology focuses to recognize the associated cardiovascular adverse effects of cancer treatment and ensure optimal interdisciplinary chemotherapy. Research efforts have produced a deep understanding of the underlying clinical relevance and pathogenesis, but standard guidelines and structural requirements for the treatment of cardiovascular malignancies remain limited [12,25,32].
The diagnosis of cardiac problems associated with cancer treatment is complex. Population heterogeneity makes it difficult to compare groups and between groups. It is often impossible to conduct large enough population studies, and the number of new drugs used to treat other diseases makes it difficult to evaluate large populations. Identify patients related to cancer treatments, who are at higher risk of cardiovascular problems or who have symptoms on one side. Post-treatment effects are an important aspect of a growing field known as cardio-oncology. Oncologists, in collaboration with cardiologists, can provide the assistance needed by those who are primarily clinicians treating cancer patients in order to improve therapy. The aim should be to augment meaningful life in every way imaginable. Excessive concern about potentially reversible heart issues can hinder the delivery of highly successful cancer treatments, while underestimating cardiac risk in a cancer-free patient can result in lifelong heart problems, a careful balance requires careful consideration of the needs of these diverse patients. The best chance of long-term disease-free survival for these individuals is to be aware of the cardiac effects of anticancer drugs, in consistent with knowledge of the normal course of malignancy and the risk of a tumour reaction [32].
Some cardiovascular mechanisms have become clearer as a result of research into the negative effects of newly emerging cancer therapies. The erbB2/ HER2 / neuregulin signaling pathway, which has been the focus of many cancer treatments, is thought to play a role in cardiovascular homeostasis, and research is underway to evaluate stimulation of this pathway may benefit patients with heart failure. Intense studies and ongoing clinical trials in oncology are the subject of signal inhibitors, chemotherapeutic agents, and combinations of these [33]. The cardiovascular adverse effects of these drugs should be expected based on their direct effect on signal transduction or the potential for additional non-targeted inhibitory effects [34]. Specific topics and priorities highlight the current state of the field as presented in each conference and to the extent possible, strategies to move the field forward in a targeted manner [35]. Since clinical and basic research data inform the analysis of our clinical practice in so many ways that need to be developed, the aim is to help identify the key knowledge gaps that need to be filled in order to understanding and improved clinical care for our patients.
They highlighted the importance of a cardio-oncology programme in the prevention, early detection and reduction of the impact of cancer treatment on the cardiovascular system in hospital and community settings. Given the rising number of FDA (Food and Drug Administration) approved anti-cancer agents with possible cardiotoxic effects, better screening before beginning anti-cancer therapy is crucial to avoiding cardiotoxicity in cancer and heart disease with similar risk factors. Clinicians are invited to identify what preventive measures should be used to reduce the risk of cardiotoxicity in patients at risk of developing cardiac dysfunction prior to initiation of therapy, during treatment with potentially cardiotoxic anti-cancer agents, and to evaluate preferred approaches to monitoring and follow-up after treatment. Cardiooncology is also considered consistent with cancer therapy for the treatment of cardiovascular toxicity; it is important to note that there are many common risk factors and pathological mechanisms between cancer and cardiac disease at the cellular and molecular levels that can be easily detected by a clinical pharmacist [36]. Cardiac toxicities due to cancer treatment include heart failure, cardiac ischemia, arrhythmias, pericarditis, valvular disease, and fibrosis of the pericardium and myocardium [37].
Although cardio-oncology departments have been established in parts of Europe and the United States, it is still a relatively new concept in the United Kingdom and many other countries, but a clear clinical need is leading several hospitals to develop standard cardio-oncology services such as Bart’s Heart Center [38], St Bartholomew’s Hospital in London, and University College London Hospital [39], and this issue is also reflected in several recent Cardio-Oncology guidelines. Cancer patients may present with a variety of cardiovascular problems that are not all directly related to anticancer therapy (drugs or radiotherapy) [25,40]. Optimal individual management requires a close collaboration between cardiology and oncology specialists.
Conclusion
Cardiac toxicity is a problem that affects cancer patients’ quality of life and well-being. To develop preventative measures based on the early identification and preventative measures of cardiotoxicity, a thorough evaluation of these patients’ needs is needed. To standardize the treatment process, cardio-oncology multidisciplinary teams must combine their abilities and skills. Also, a clinical pharmacist should be integrated with the multidisciplinary team responsible for the treatment of cancer patients to evaluate the potential drug interactions. This recognition has aided in the stimulation of collaboration among oncologists, cardiologists, and researchers, and integrated discipline of cardio-oncology can have the results in many medical centers. This collaboration aims perception into how cancer treatments lead to long-term effects on cancer patients and cardiovascular homeostasis. The most recent perspectives on cardiotoxicity with different classes of chemotherapeutic agents and drug-drug interactions with cardiovascular drugs in cancer patients with pre-existing cardiovascular diseases were discussed in this review. We also discussed the importance, role, and necessity of a cardiooncologist.
Conflict of Interest
Authors declare that they have no conflict of interests
Acknowledgments
We take this valuable opportunity to convey our gratitude to Dr. S.A Azeez, Principal, Deccan School of Pharmacy and Prof. Dr. Osman Ahmed, Vice Principal, Professor, Deccan School of Pharmacy for granting essential facilities, suggestions and inspiration.
References
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (1967) Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 20(3): 333-353.
- Koelwyn GJ, Jones LW, Moslehi J (2014) Unravelling the causes of reduced peak oxygen consumption in patients with cancer: complex, timely, and necessary. J Am Coll Cardiol. 64(13): 1320-1322.
- Gharib MI, Burnett AK (2002) Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail. 4(3): 235-242.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 103(2): 117-128.
- Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, et al. (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 107(9): 1375-1380.
- Geisberg CA, Abdallah WM, Silva M, Silverstein C, Smith HM, et al. (2013) Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J Card Fail 19(1): 10-15.
- Cardinale D, Sandri MT (2010) Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 53(2): 121-129.
- Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T (2019) Cardio-oncology- strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 280: 163-175.
- Snipelisky D, Park JY, Lerman A, Mulvagh S, Lin G, et al. (2017) How to develop a cardio-oncology clinic. Heart Fail Clin. 13(2): 347-359.
- Levis BE, Binkley PF, Shapiro CL (2017) Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol 18(8): e445-e456.
- Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, et al. (2018) Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 137(8): e30-e66.
- Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH (2017) Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol 70(20): 2536-2551.
- Zamorano JL, Lancellotti P, Munoz DR, Aboyans V, Asteggiano R, et al. (2016) 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37(36): 2768-2801.
- Barish R, Lynce F, Unger K, Barac A (2019) Management of cardiovascular disease in women with breast cancer. Circulation 139(8): 1110-1120.
- Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].
- Elitok A, Oz F, Cizgici AY, Kilic L, Ciftci R, et al. (2014) Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J 21(5): 509-515.
- Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, et al. (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63(25 Pt A): 2751-2768.
- Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, et al. (2018) Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society-one trial. Eur J Cancer 94: 126-137.
- Gyenes G, Fornander T, Carlens P, Glas U, Rutqvist LE (1997) Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur J Nucl Med. 24(3): 286-292.
- Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC (1993) Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 69(6): 496-500.
- Brosius FC III, Waller BF, Roberts WC (1981) Radiation heart disease: analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 70(3): 519-530.
- Veinot JP, Edwards WD (1996) Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 27(8): 766-773.
- Chello M, Mastroroberto P, Romano R, Zofrea S, Bevacqua I, et al. (1996) Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovasc Surg. 4(2): 222-226.
- Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, et al. (2004) Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 109(25): 3122-3131.
- Lancellotti P, Suter TM, Fernández LT, Galderisi M, Lyon AR, et al. (2019) Cardio-Oncology Services: rationale, organization, and implementation. Eur Heart J 40(22): 1756-1763.
- Leeuwen RW, Brundel DH, Neef C, Gelder T, Mathijssen RH, et al. (2013) Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer. 108(5): 1071-1078.
- Lenneman CG, Sawyer DB (2016) Cardio-Oncology: An update on cardiotoxicity of cancer-related treatment. Circ Res 118(6): 1008-1020.
- Kennedy C, Brewer L, Williams D (2016) Drug interactions. Medicine 44: 422-426.
- Tenkoramaa SJ, Fudin J (2013) Drug Interactions in Cancer Patients Requiring Concomitant Chemotherapy and Analgesics. Prac Pain Manag 13(4): 50-64.
- Heron M (2018) Deaths: Leading Causes for 2016. Natl Vital Stat Rep. 67(6): 1-77.
- Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 25(7):1029-1036.
- Suter TM, Ewer MS (2013) Cancer drugs and the heart: importance and management. Eur Heart J. 34(15)P: 1102-1111.
- Lemmens K, Doggen K, Keulenaer GW (2007) Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 116(8): 954-960.
- Eschenhagen T, Force T, Ewer MS, Keulenaer GW, Suter TM, et al. (2011) Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 13(1):1-10.
- Force T, Krause DS, Van Etten RA (2007) Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 7(5): 332-344.
- Fadol AP, Palaskas NL, Ewer MS Deswal A (2020) An overview of a different type of cardio-oncology gathering: summary of the COMP (cardio-oncology multidisciplinary practice) meeting held in Houston Texas, January 2020. Cardio-Oncology 6:20.
- Ewer M, Ewer S (2015) Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 12(9): 547-558.
- Lenihan DJ, Cardinale DM (2012) Late cardiac effects of cancer treatment. J Clin Oncol. 30(30): 3657-3664.
- Ghosh AK, Manisty C, Woldman S, Crake T, Westwood M, et al. (2017) Setting up cardio-oncology services, Br J Cardiol, 24(1): 1-5.
- Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, et al. (2017) Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 35(8):893-911.
© 2022 Misbah Ul Haq M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






