- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Atrial Tachycardias After Catheter Ablation of Atrial Fibrillation
Tampakis Konstantinos*
Department of Electrophysiology & Pacing, Henry Dunant Hospital Center, Athens, Greece
*Corresponding author: Tampakis Konstantinos, Department of Electrophysiology & Pacing, Henry Dunant Hospital Center, Athens, Greece
Submission: February 18, 2022;Published: March 04, 2022

ISSN 2578-0204Volume3 Issue4
Abstract
Atrial tachycardias after catheter ablation of atrial fibrillation are a significant complication as these arrhythmias are often more symptomatic than index arrhythmia and require a repeat ablation procedure for long-term maintenance of sinus rhythm. The reported incidence ranges from <5% to 50% with an increased risk when atrial fibrillation duration is prolonged and when extensive lesions are performed, in addition to pulmonary vein isolation, during the index ablation procedure. Macro-reentry is the most common mechanism while localized reentry represents the majority of focal atrial tachycardias. Electroanatomical mapping using a stepwise approach is the cornerstone for evaluation of the underlying mechanism and ablation guidance. For focal tachycardias, ablation targets the site of earliest activation that usually involves a reconnected pulmonary vein ostium. In localized reentry, localization is based on identification of electrogram fractionation covering a great proportion of the cycle length within a small area while linear ablation is the approach of choice for the most frequent macro-reentrant atrial tachycardias.
Keywords: Atrial tachycardia; Catheter ablation; Macro-reentry; Localized reentry; Atrial fibrillation ablation
Introduction
Catheter ablation is a technique that has been established as one of the main treatment modalities and is increasingly applied in patients with Atrial Fibrillation (AF) [1]. However, post-ablation Atrial Tachycardias (ATs) are a significant complication as these arrhythmias are often more symptomatic than index AF, respond poorly to medical therapy or electrical cardioversion and thus a repeat ablation procedure is frequently required for long-term maintenance of sinus rhythm [2].
Incidence
The reported incidence of AT after AF ablation ranges from <5% to 50% with an increased risk when AF duration is prolonged and when extensive lesions are performed during the index ablation procedure in addition to Pulmonary Vein Isolation (PVI) [3]. Electrical isolation of the Pulmonary Veins (PVs) is widely accepted as the cornerstone of catheter ablation for AF [1]. Incidence of ATs after PVI has been variably reported from 2.9% to 10% [4,5]. Isolation of a large circumferential area around both ipsilateral PVs is increasingly performed as this approach has been demonstrated more effective than segmental isolation of each PV [6]. However, circumferential PVI is also associated with a significantly higher incidence of ATs than segmental isolation [5]. In patients with persistent AF, additionally to PVI, substrate modification was often performed, mainly linear lesions in the left atrium and focal ablation to eliminate complex fractionated electrograms. However, the results of the randomized Substrate and Trigger Ablation for Reduction of AF Trial Part II (STAR AF II) trial failed to demonstrate any reduction in the rate of recurrent AF [7]. On the other hand, the highest incidence of ATs (as high as 50%) has been reported in persistent AF patients undergoing substrate ablation, especially a more extensive ablation involving PVI, linear lesions and electrogram based ablation [8]. The majority of ATs after AF ablation occurs during the blanking period (the 3-month post-ablation period), especially the first month, and also predicts late AT or recurrence of AF [9,10]. ATs that persist after the blanking period or occur late after AF ablation usually require a repeat intervention [9].
Mechanism
Post-ablation ATs were classified for long into two categories: macro-reentrant and focal [11]. More recently, a distinct mechanism, localized reentry, was recognized to represent the majority of the latter [10]. It has been observed that the AT mechanism is usually related to the prior ablation technique while multiple ATs may be observed frequently [12]. The majority of left ATs in previous PVI are usually related to gaps in prior circumferential or segmental ablation lines or slow conduction adjacent to the PV lesions [13]. Focal AT has been found to account for 12% to 22.2% of ATs after PVI and re-isolation alone is sufficient in their termination [6,14]. However, most ATs after PVI are of reentrant mechanism as a result of non-contiguous previous ablation [14]. On the other hand, ATs after durable PVI are less common (Figure 1). Localized reentry involves a small region of slow conduction sustaining entire circuit locally within < 2cm diameter and a centrifugal activation of the left atrium [10]. Most commonly these regions are at the PV ostia (Figure 2) and/or in relation to previous lesions in case of extended ablation. Macro-reentry is the most common mechanism of post-ablation ATs, especially after wide antral PV ablation or linear lesions [12]. More than half of recurrent ATs are macroreentrant and up to 72% after ablation in persistent AF [15,16]. A component of the reentry circuit usually traverses a prior ablation line, indicating a gap-related mechanism [14]. Among the macroreentrant ATs, perimitral flutter is the most frequent, followed by roof-dependent flutter
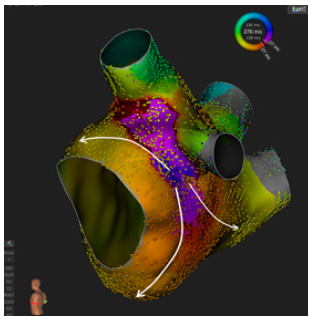
Figure 1:Activation mapping of a roof-dependent atrial flutter after durable pulmonary vein isolation with cryoballoon ablation.
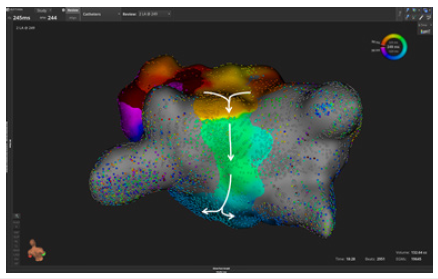
Figure 2:Activation mapping of a localized reentrant atrial tachycardia after pulmonary vein isolation with radiofrequency ablation. A centrifugal activation of the left atrium is observed.
Electroanatomical Mapping and Ablation
ECG features as flutter waves remain useful to differentiate focal from macroreentrant Ats [17]. However, proposed algorithms may be unreliable after extensive ablation of the left atrium [18].
In contrast with ECG, electrophysiological study is the most reliable tool for the diagnosis of the underlying mechanism. A mapping strategy of three steps has been proposed for evaluation of post-ablation ATs during ongoing tachycardia [10]. Assessment of AT cycle length stability should be the first step. In cases of cycle length variability of greater than 15%, a focal mechanism is very likely. Secondly, when AT cycle length does not present irregularity, classical entrainment manoeuvres and activation mapping are useful to diagnose or exclude macro-reentry and to clarify sites that participate in a circuit. The third step includes identification of the location in case a focal mechanism is suggested. Moreover, PV reconnections should be identified and followed by re-isolation, usually before targeting other regions. Novel mapping tools may also have an important role in the diagnosis of more challenging cases [19]. For focal ATs, ablation targets the site of earliest activation that usually involves a reconnected PV ostium [20]. For the majority of reentrant ATs occurring after ablation for AF, focally narrow isthmuses of slow conduction are critical [4]. In localized reentry, electrogram fractionation covering a great proportion of the cycle length of AT is presented within a small area and guides ablation [10]. Macro-reentrant ATs are targeted by performing linear ablation connecting the nearest anatomical or electrical barriers. A mitral isthmus line, usually posterolateral, from the mitral annulus to the left inferior PV, is performed in case of perimitral flutter. Epicardial ablation inside the coronary sinus or by injecting ethanol into the vein of Marshall may also be required [21]. An anterior line is an alternative approach that might be considered when extensive scar of the anterior left atrium is presented [22]. The roof line, between the superior PVs, is typically performed for the second most common roof-dependent flutter while in more complex arrhythmias the critical isthmus should be identified. In the end of the procedure, consideration should be given to confirming complete conduction block of linear lesions in order to avoid recurrences.
Conclusion
ATs after ablation for AF are a significant complication as these arrhythmias are often symptomatic and require a repeat ablation. Their prevalence may be reduced by avoiding routine substrate ablation during the initial procedure. Electroanatomical mapping is the cornerstone for evaluation of the underlying mechanism and ablation guidance.
References
- Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, et al. (2018) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 20(1): e1-e160.
- Daoud EG, Weiss R, Augostini R, Hummel JD, Kalbfleisch SJ, et al. (2006) Proarrhythmia of circumferential left atrial lesions for management of atrial fibrillation. J Cardiovasc Electrophysiol 17(2): 157-165.
- Sawhney N, Anousheh R, Chen W, Feld GK (2010) Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 3(3): 243-248.
- Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, et al. (2005) Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: A randomized comparison between 2 current ablation strategies. Circulation 111(22): 2875-2880.
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, et al. (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372(19): 1812-1822.
- Gerstenfeld EP, Callans DJ, Dixit S, Russo AM, Nayak H, et al. (2004) Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 110(11): 1351-1357.
- Matsuo S, Lim KT, Haissaguerre M (2007) Ablation of chronic atrial fibrillation. Heart Rhythm 4(11): 1461-1463.
- Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, et al. (2008) Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 5(5): 679-685.
- Baman JR, Kaplan RM, Diaz CL, Peigh G, Bavishi AA, et al. (2020) Characterization of atrial flutter after pulmonary vein isolation by cryoballoon ablation. J Interv Card Electrophysiol 57(2): 233-240.
- Jaïs P, Matsuo S, Knecht S, Weerasooriya R, Hocini M, et al. (2009) A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: Importance of localized reentry. J Cardiovasc Electrophysiol 20(5): 480-491.
- Saoudi N, Cosío F, Waldo A, Chen SA, Iesaka Y, et al. (2001) A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a statement from a joint expert group from the working group of arrhythmias of the European society of cardiology and the North American society of pacing and electrophysiology. Eur Heart J 22(14): 1162-1182.
- Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, et al. (2005) Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 16(11): 1138-1147.
- Chang SL, Lin YJ, Tai CT, Lo LW, Tuan TC, et al. (2009) Induced atrial tachycardia after circumferential pulmonary vein isolation of paroxysmal atrial fibrillation: Electrophysiological characteristics and impact of catheter ablation on the follow-up results. J Cardiovasc Electrophysiol 20(4): 388-94.
- Chae S, Oral H, Good E, Dey S, Wimmer A, et al. (2007) Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 50(18): 1781-1787.
- Rostock T, Drewitz I, Steven D, Hoffmann BA, Salukhe TV, et al. (2010) Characterization, mapping, and catheter ablation of recurrent atrial tachycardias after stepwise ablation of long-lasting persistent atrial fibrillation. Circ Arrhythm Electrophysiol 3(2): 160-169.
- Pascale P, Shah AJ, Roten L, Scherr D, Komatsu Y, et al. (2013) Pattern and timing of the coronary sinus activation to guide rapid diagnosis of atrial tachycardia after atrial fibrillation ablation. Circ Arrhythm Electrophysiol 6(3): 481-490.
- Chang SL, Tsao HM, Lin YJ, Lo LW, Hu YF, et al. (2011) Differentiating macroreentrant from focal atrial tachycardias occurred after circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol 22(7): 748-755.
- Gerstenfeld EP, Dixit S, Bala R, Callans DJ, Lin D, et al. (2007) Surface electrocardiogram characteristics of atrial tachycardias occurring after pulmonary vein isolation. Heart Rhythm 4(9): 1136-1143.
- Margato R, Tampakis K, Albenque JP, Combes S (2020) Illuminating the marshall: Novel techniques highlighted in an atrial tachycardia case report. Eur Heart J Case Rep 4(4): 1-5.
- Cummings JE, Schweikert R, Saliba W, Hao S, Martin DO, et al. (2005) Left atrial flutter following pulmonary vein antrum isolation with radiofrequency energy: Linear lesions or repeat isolation. J Cardiovasc Electrophysiol 16(3): 293-297.
- Kitamura T, Vlachos K, Denis A, Andre C, Martinet R, et al. (2019) Ethanol infusion for marshall bundle epicardial connections in marshall bundle-related atrial tachycardias following atrial fibrillation ablation: The accessibility and success rate of ethanol infusion by using a femoral approach. J Cardiovasc Electrophysiol 30(9): 1443-1451.
- Huemer M, Wutzler A, Parwani AS, Attanasio P, Matsuda H, et al. (2015) Comparison of the anterior and posterior mitral isthmus ablation lines in patients with perimitral annulus flutter or persistent atrial fibrillation. J Interv Card Electrophysiol 44(2): 119-129.
© 2022 Tampakis Konstantinos. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






