- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
The Incidence of N1 And N2 Metastases in Relation to the Presence/Absence of the Peritumoral Lymphovascular Invasion
Pilav I1 , Mušanović S1 *, Karavdić K2 , Alihodžić-Pašalić A1 , Dapčević M1 , and Čustović O1
1Department of Thoracic Surgery, Bosnia and Herzegovina
2Department of Pediatric Surgery, Bosnia and Herzegovina
*Corresponding author: Mušanović S, Department of Thoracic Surgery, Bosnia and Herzegovina
Submission: June 23, 2020;Published: January 27, 2021

ISSN 2578-0204Volume3 Issue3
Abstract
Introduction: The incidence of N1 and N2 metastases in relation to the presence of peritumoral lymphovascular infiltration and tumor grade in bronchial carcinoma still remain insufficiently researched areas that could provide valuable guidance. Several studies have shown a statistically significant difference in the occurrence of N1 and N2 metastases in relation to the type and size of the bronchial carcinoma. The relationship between the occurrence of N1 and N2 metastases and the degree of immaturity of the tumor in the bronchial carcinoma remains insufficiently documented.
Patients and methods: This study included 331 patients of all ages, both men and women, diagnosed with bronchial carcinoma using various diagnostic procedures. In these patients, surgical treatment was indicated and the anatomical resection was performed.
Result: A total of 331 patients were included in the study and N1 metastases were present in 39.88% of cases, while N2 metastases were present in 4.53% of cases with bronchial carcinoma. Peritumoral lymphovascular invasion was present in 37.55% of cases (86 out of 229 patients) with N0 disease, in 55.89% of cases (128 out of 229 patients) with N1 disease, and in 6.55% of cases (15 out of 229 patients) with confirmed N2 disease.
Conclusion: The presence of the peritumoral lymphovascular invasion is more often accompanied by metastases in corresponding regional lymph nodes. By calculating the total relative risk, there is almost a 30-fold higher risk of developing metastases in N1 and N2 lymph nodes in the presence of peritumoral lymphovascular invasion.
Keywords: Bronchial carcinoma; Lymph node metastases; Lymphovascular invasion
Introduction
The incidence of N1 and N2 metastases in relation to the presence of peritumoral
lymphovascular infiltration and tumor grade in bronchial carcinoma still remain insufficiently
researched areas that can provide valuable guidance on cancer aggressiveness, disease spread,
prognosis and treatment.
Being that each lung lobe has its own lymphatic drainage, metastatic spread of lung cancer
by lymphatic route depends on its location. Consequently, understanding the metastatic
spread of cancer to regional lymph nodes requires good knowledge of the anatomy of the
lymphatic system of each lobe [1-6].
The influence of numerous factors, i.e., the most common types of lung cancer, tumor size
as well as the degree of differentiation and stage of the malignant disease on the occurrence
of N1 and N2 metastases has been thoroughly investigated. Several studies have shown a
statistically significant difference in the occurrence of N1 and N2 metastases in relation to the
type and size of the bronchial carcinoma. The relationship between the occurrence of N1 and
N2 metastases and the degree of immaturity of the tumor in the bronchial carcinoma remains
insufficiently documented [1-6].
A literature review of available medical databases and research articles over the past
10 years was conducted, but the strong association between the presence of N1 and N2 metastases and pathohistological confirmation of peritumoral
lymphovascular invasion (a reliable indicator that correlates
with the tumor aggressiveness in bronchial carcinoma) was not
observed in other studies.
Patients and Methods
We analyzed data from patients diagnosed with bronchial carcinoma who underwent surgical resection during their hospital stay at the Clinic of Thoracic Surgery, Clinical Center University of Sarajevo from 01.01.2013 to 01.01.2018. In total, this study included 331 patients of all ages, both men and women, diagnosed with bronchial carcinoma using various diagnostic procedures. In these patients, surgical treatment was indicated, and the anatomical resection was performed.
Results
A total of 331 patients diagnosed with bronchial carcinoma
underwent surgical resection during their hospital stay at the Clinic
of Thoracic Surgery from 01.01.2013 to 01.01.2018. The mean age
of the patients was 62.69 ± 7.46.
Histopathological findings in relation to the type of bronchial
carcinoma are shown in Table 1.
Table 1:Definitive histopathological diagnoses in relation to the type of bronchial carcinoma.
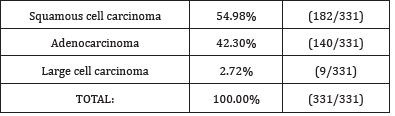
In our study, the squamous cell carcinoma was the most common
type of bronchial carcinoma, found in 54.98% of cases (182 out of
331 patients), followed by adenocarcinoma, which was found in
42.30% of cases (140 out of 331 patients). The least common type
of bronchial carcinoma was large cell carcinoma, found in 2.72% of
cases (nine out of 331 patents).
The relation between the most common histopathological
diagnoses and surgical resections is shown in Figure 1.
Figure 1:The relation between the most common histopathological diagnoses and surgical resections.
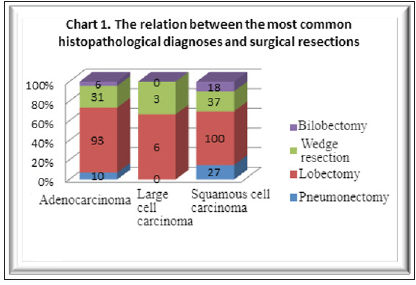
Standard lobectomy was the most common surgical resection
of both squamous cell carcinoma, i.e., in 54.94% of cases (100 out
of 182 patients) and adenocarcinoma, i.e., 66.42% of cases (93 out
of 140 patients).
The prevalence of lymph node (N0, N1 and N2) involvement in
bronchial carcinoma is shown in Table 2.
Table 2:The prevalence of lymph node involvement in patients diagnosed with bronchial carcinoma.
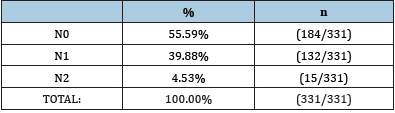
The most common were cases without the presence of
malignant cells in the lymph nodes (N0), i.e., 55.59% of cases (184
out of 331 patients), N1 metastases were present in 39.88% of
cases (132 out of 331 patients), and N2 metastases were observed
in 4.53% of cases (15 out of 331 patients).
The relation between N0, N1 and N2 metastases and the most
common histopathological diagnoses is shown in Table 3.
Table 3: The prevalence of N0, N1 and N2 metastases and their relation to the most common histopathological diagnoses.

More than half of the patients with both squamous cell
carcinoma, i.e., 52.75% of cases (96 out of 182 patients) and
adenocarcinoma, i.e., 58.57% of cases (82 out of 140 patients)
had no lymph node metastases (N0). N1 lymph node involvement
(positive ipsilateral hilar lymph nodes) was present in 43.96% of
cases with squamous cell carcinoma and in 35.00% of cases with
adenocarcinoma. N2 lymph node involvement was present in
3.30% of cases with squamous cell carcinoma and in 6.43% of cases
with adenocarcinoma.
The prevalence of the peritumoral lymphovascular invasion
and its relation to the lymph node status is shown in Table 4.
Table 4: The prevalence of the peritumoral lymphovascular invasion and its relation to the lymph node status.
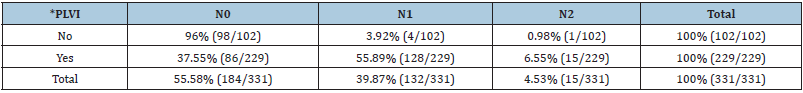
*PLVI - peritumoral lymphovascular invasion.
Peritumoral lymphovascular invasion was present in 37.55% of
cases (86 out of 229 patients) with N0 disease and in 55.89% of
cases (128 out of 229 patients) with N1 disease as well as in 6.55%
of cases (15 out of 229 patients) with confirmed N2 disease.
Peritumoral lymphovascular invasion was not present in 96%
of cases (98 out of 102 patients) with NO disease, 3.92% of cases
(4 out of 102 patients) with N1 disease as well as 0.98% of cases (1
out of 102 patients) with confirmed N2 disease.
Table 5: The frequency of lymph node metastases in relation to the presence of peritumoral lymphovascular invasion.
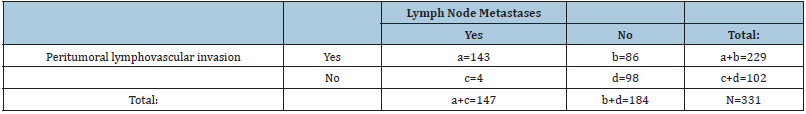
The frequency of lymph node metastases in relation to the
presence of peritumoral lymphovascular invasion is shown in Table
5.
It is possible to calculate the sensitivity, specificity, positive
and negative predictive values of the peritumoral lymphovascular
infiltration in relation to the presence of lymph node metastases.
Their values were obtained as follows: sensitivity (97.27%),
specificity (53.26%), positive predictive value (62.447%) and
negative predictive value (96.07%). The overall accuracy was
72.8%.
Table 6: The prevalence of N1 lymph node metastases in relation to the presence of peritumoral lymphovascular infiltration.

Data for calculating the relative risk of N1 lymph node metastases
in relation to the presence of peritumoral lymphovascular
infiltration is shown in Table 6.
The risk of lymph node metastases in patients with peritumoral
lymphovascular invasion was calculated as follows:
The risk among patients with PLVI = (a/b) = 128/4 = 31.00.
The risk of lymph node metastases in patients without
peritumoral lymphovascular invasion was calculated as follows:
The risk among patients without PLVI = (c/d) = 86/98 = 0.87
We investigated the association between the peritumoral
lymphovascular invasion and regional lymph node metastases using
Spearman’s rank correlation coefficient. There was a statistically
significant correlation between the variables, i.e., ρ = 0.544, n = 331,
p < 0.0001. The presence of peritumoral lymphovascular invasion
was more often accompanied by the presence of metastases in the
corresponding regional lymph nodes.
Discussion
The presence of N1 disease significantly affects the five-year
survival rate, and it has been observed that the same corresponds
to the number of involved N1 lymph nodes. The presence of N2
disease is still the subject of much debate, which leads to the fact
that most surgeons and oncologists at present opt for a combined
approach to N2 NSCLC. It has been shown that patients who have
been preoperatively diagnosed with N2 lymphadenopathy do not
benefit much from surgical treatment [7-10]. In contrast, patients
whose N2 disease is not clearly clinically classified, but diagnosed
via surgical treatment or using the histopathological examination,
have a better postoperative outcome [11,12].
Lymph nodes in patients diagnosed with bronchial carcinoma
who were treated in this study were not proportionally affected by
metastatic spread of the disease as seen from the obtained results.
The most common were patients without the presence of malignant
cells in the lymph nodes (N0), i.e., 55.59% of cases, N1 metastases
were present in 39.88% of cases, and N2 metastases in 4.53% of
cases of bronchial carcinoma.
Almost similar results have been published in several other
literature reports [1-9]. Given the fact that the majority, i.e., 95.47%,
of patients who have undergone surgical treatment had N0 and N1
lymph node status, it can be assumed and concluded that there
was a relatively good diagnostic assessment of patients as well as
clinical determination of the disease stage and selection of patients
for surgical treatment.
In more than half of the patients, both in the squamous cell
population group, i.e., 52.75% of cases (96 out of 182 patients)
as well as in the adenocarcinoma population group, i.e., 58.57%
of cases (82 out of 140 patients), no lymph node metastases were
present.
In relation to the presence or absence of peritumoral
lymphovascular invasion in patients diagnosed with bronchial
carcinoma, the following results were obtained:
Peritumoral lymphovascular invasion was present in 72.46% of
cases (229 out of 316 patients) with the presence of lymph node
metastases in 62.44% of cases [N1 metastases in 89.51% of cases
(128 out of 143 patients) and N2 in 10.49% of cases (15 out of 143
patients)].
Based on the available data, sensitivity (97.27%), specificity
(53.26%), positive predictive value (62.447%) as well as the
negative predictive value (96.07%) were calculated. The overall
accuracy was 72.8%.
The total relative risk of lymph node metastases in relation
to the presence of peritumoral lymphovascular invasion is
35.63, which means that there is a 35.63 times higher chance of
developing disease with the lymph node metastasis in the presence
of peritumoral lymphovascular invasion compared to when it is not
present.
The study unequivocally confirmed that the presence of
peritumoral lymphovascular invasion was more often accompanied
by the presence of metastases in the corresponding regional
lymph nodes. An association between the presence of peritumoral
lymphovascular invasion and the frequency of N1 and N2
metastases was observed.
The presence of peritumoral lymphovascular invasion can
serve as a good predictor in relation to the assessment of the lymph
node status, and indirectly have both prognostic and therapeutic
significance for these patients.
However, there are reports in the available literature on other
types of malignancies, primarily breast cancer and cervical cancer, in
which similar studies (the presence of peritumoral lymphovascular
invasion and the lymph node status) have been conducted and have
shown an association between the peritumoral lymphovascular
invasion and more frequent occurrence of lymph node metastases
[7-13].
Conclusion
a) Peritumoral lymphovascular invasion was present in 70.52%
of cases (229 out of 331 patients) diagnosed with bronchial
carcinoma.
b) Lymph nodes were involved in 70.52% of cases (229 out of
331 patients) with present lymphovascular invasion and
in only 3.30% of cases (4 out of 331 patients) in which the
lymphovascular invasion was not present.
c) Lymph nodes were not involved in 84% of cases (86 out of 229
patients) with present lymphovascular invasion and in 53.00%
of cases (98 out of 102 patients) in which the lymphovascular
invasion was not present.
d) In those cases, without lymph node involvement (N0)
peritumoral lymphovascular invasion was present in 46.13%
of cases (86 out of 244 patients).
e) Peritumoral lymphovascular invasion was present in 53.26%
of cases (143 out of 244 patients) with confirmed N1 disease.
f) Peritumoral lymphovascular invasion was present in 100% of
cases (all 15 patients) with confirmed N2 disease.
g) Regardless of the fact that the presence of the peritumoral
lymphovascular invasion was not accompanied by the
occurence of N1 and N2 metastases in all patients diagnosed
with bronchial carcinoma, it was found that there was a
statistically significant difference in metastatic lymph node
involvement in relation to the presence of the peritumoral
lymphovascular invasion.
h) By calculating the total relative risk, there is almost a 30-fold
higher risk of developing metastases in N1 and N2 lymph nodes
in the presence of the peritumoral lymphovascular invasion.
The presence or absence of the peritumoral lymphovascular invasion can be used both in predicting and excluding the presence or absence of the lymph node involvement.
References
- (2009) Cancer facts and figures. American Cancer Society.
- Shields TW (2005) Pathology of carcinoma of the lung. In: General Thoracic Surgery. (6th edn), Lippincott Williams and Wilkins, USA, pp. 1455-1480.
- Mostertz W, Stevenson M, Acharya C, Chan I, Walters K, et al. (2010) Age-and sex-specific genomic profiles in non-small cell lung cancer. JAMA 303(6): 535-543.
- Porta RR, Crowley JJ, Goldstraw P (2009) The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 15(1): 4-9.
- De la Guerra A (2010) New TNM classification for lung cancer - Part I: The changes.
- Padilla J, Calvo V, Penalver JC, Sales G, Morcillo A (1997) Surgical results and prognostic factors in early non-small cell lung cancer. Ann Thorac Surg 63(2): 324-326.
- Lantuejoul S, Brambilla E (2006) Prognostic biomarkers in non-small cell lung carcinoma. Curr Diagn Pathol 2: 418-428.
- Sakao Y, Nakazono T, Sakuragi T, Natsuaki M, Itoh T (2004) Predictive factors for survival in surgically resected clinical IA perpiheral adenocarcinoma of the lung. Ann Thorac Surg 77(4): 1157-1162.
- (2009) National Comprehensive Cancer Network. NSCLC Practice Guidelines.
- Marra A, Hillejan L, Zaboura G, Fujimoto T, Greschuchna D, et al. (2003) Pathologic N1 non-small cell lung cancer: Correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg 125(3): 543-553.
- Heerma van Voss MR, vander Groep P, Bart J, vander Wall E, van Diest (2010) Lymphovascular invasion in BRCA related breast cancer compared to sporadic controls. BMC Cancer 10: 145.
- Parsons A, Daley A, Begh R, Aveyard P (2010) Influence of smoking cessation after diagnosis of early-stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 340: b5569.
- Yoshino I, Baba H, Fukuyama S, Kameyama T, Shikada Y, et al. (2002) A time trend of profile and surgical results in 1123 patients with non-small lung cancer. Surgery 131: S242-S428.
© 2021 Mušanović S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






