- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Effect of HER2 Receptor Expression on Cardiac Ejection Fraction in Cancer Patients Treated with Trastuzumab
Cole Lightfoot*, Carolyn Oxencis, Elizabeth Weil, Angela Urmanski, Briana Amundson, Lisa Rein and Sailaja Kamaraju
Froedtert & the Medical College of Wisconsin, Department of Pharmacy, USA
*Corresponding author: Cole Lightfoot, Froedtert Health, Department of Pharmacy, USA
Submission: September 27, 2019;Published: October 31, 2019

ISSN 2578-0204Volume3 Issue1
Abstract
Background: Human epidermal growth factor receptor 2 (HER2) over expression has been identified as an important drug target for the treatment of many cancers. Trastuzumab (Herceptin, Genentech, CA, USA) is an anti-HER2 monoclonal antibody effective at inducing cell death in patients who overexpress this gene. The degree of HER2 expression is a determining factor in the decision to treat patients with trastuzumab. At Froedtert & the Medical College of Wisconsin, a dual probe fluorescent in situ hybridization is completed and the decision to treat is then made based on the results which are reported as a ratio of HER2 gene signals relative to those of chromosome 17 (CEP17). A patient may qualify for treatment by meeting the minimum threshold requirement of HER2 expression (HER2:CEP17 ratio ≥2 or if HER2:CEP17 ratio <2 and ≥6 HER2 signals/cell), while other patients may far surpass the minimum level. Trastuzumab contains a warning for possible cardiomyopathy associated with symptomatic and asymptomatic reductions in left ventricular ejection fraction (LVEF). There has been no investigation as to whether the degree of HER2 expression is related to the patient’s risk for developing trastuzumab-induced cardiotoxicity.
Methods: We performed a retrospective cohort review of patients having received trastuzumab within Froedtert & the Medical College of Wisconsin Health System in an effort to identify and describe the relationship between degree of HER2 expression and incidence of cardiotoxicity in patients receiving trastuzumab therapy. The primary objective of this study was to identify the correlation between HER2 receptor expression and cardiotoxicity in patients treated with trastuzumab. The secondary objectives were to identify the correlation between HER2 expression and absolute change in LVEF and time to cardiotoxicity in patients treated with trastuzumab. For the purposes of this study, cardiotoxicity was defined as a decrease in LVEF ≥ 16% from baseline or a LVEF <50% and ≥10% decrease from baseline. Change in global peak systolic longitudinal strain of ≥15%, grade ≥3 left ventricular systolic dysfunction as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events v 4.03 (NCI CTCAE), or treatment discontinuation due to trastuzumab cardiotoxicity as determined by physician was also used to meet criteria for primary end point of cardiotoxicity. Froedtert & the Medical College of Wisconsin IRB approved completion of this study.
Results: There were 44 (34 non-metastatic and 10 metastatic) patients with cardiotoxicity event(s) and 11 deaths without cardiotoxicity (all metastatic patients) within 18 months of follow-up. Overall one-year incidence of cardiotoxicity was 14.2% [10.7%, 18.9%], with incidences of 13.6% [9.9%, 18.8%] in non-metastatic patients and 16.7% [9.1%, 30.5%] in metastatic patients. The median survival time (time at which half of all patients are expected to experience cardiotoxicity) was not reached within 18 months. There was no significant difference in survival from cardiotoxicity between HER2:CEP17 groups (<3, 3-6, 6-9, 9+) among non-metastatic patients (log-rank test p=0.72) or metastatic patients (log-rank test p=0.17). The HER2:CEP17 ratio (continuous) was not significantly associated with cardiotoxicity in non-metastatic patients (aHR 1.00 [0.94, 1.07], p=0.95) or metastatic patients (aHR 0.88 [0.72, 1.07], p=0.19) after adjusting for baseline age, comorbidities, radiation exposure, and anthracycline exposure. In the multiple regression analysis of non-metastatic patients, anthracycline exposure (aHR 2.73 [1.28, 5.81], p=0.009), baseline comorbidities (aHR 2.41 [1.14, 5.09], p=0.021), and younger age at first dose (aHR 0.95 [0.92, 0.98], p=0.002) were significantly associated with increased hazard of cardiotoxicity. There were 56 (47 non-metastatic and 9 metastatic) patients with LVEF reduction. Overall one-year incidence of LVEF reduction was 17.4% [13.5%, 22.3%], with incidences of 18.6% [14.2%, 24.4%] in non-metastatic patients and 12.3% [6.2%, 24.8%] in metastatic patients. The median time to LVEF reduction was not reached within 18 months. The HER2:CEP17 ratio (continuous) was not significantly associated with LVEF reduction in non-metastatic patients (aHR 1.02 [0.97, 1.07], p = 0.53) or metastatic patients (aHR 0.81 [0.62, 1.05], p = 0.12) after adjusting for age, baseline comorbid conditions, radiation exposure, and anthracycline exposure (Tables 1-3). Only anthracycline exposure was significantly associated with increased hazard of LVEF reduction (aHR 2.37 [1.27, 4.43], p = 0.007) in non-metastatic patients.
Conclusion: Tumor cell HER2:CEP17 ratio does not predict incidence or time to onset of cardiotoxicity associated with trastuzumab.
Keywords: Trastuzumab; Cardiotoxicity; HER2
Abbreviations: HER2: Human Epidermal Growth Factor Receptor 2; LVEF: Left Ventricular Ejection Fraction; IHC: Immunohistochemistry; CEP17: Chromosome 17; FISH: Fluorescent Insitu Hybridization; MUGA: Multigated Acquisition Scan; NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events
Table 1:Baseline demographics.
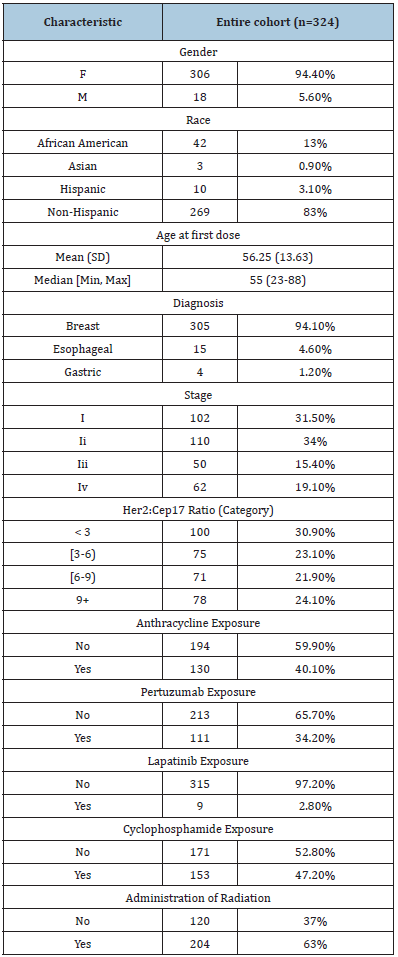
Table 2:Association with LVEF reduction in Non-Metastatic Patient Population
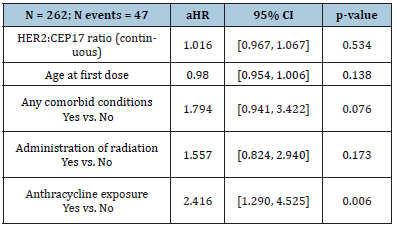
Table 3:Association with LVEF reduction in Metastatic Patient Population
Introduction
Human epidermal growth factor receptor 2 (HER2) overexpression has been identified as an important drug target for the treatment of certain malignancies. Once activated, HER2 is responsible for a signaling cascade associated with proliferation and cell survival. In 1998, trastuzumab (Herceptin®, Genentech, CA, USA) an anti-HER2 monoclonal antibody, was developed and found to be effective at inducing cell death in patient’s tumor cells which overexpress this gene [1-3]. Trastuzumab is FDA approved for use in HER2 positive breast cancers as well as in HER2 positive metastatic gastric cancer. Trastuzumab contains a black boxed warning for possible cardiomyopathy associated with reductions in left ventricular ejection fraction (LVEF). Research has shown HER2 receptor mediated signaling pathways control critical cellular functions related to cardiac myocyte survival and hypertrophy. In animal models, HER2 knockout mice developed cardiomyopathy within 12 weeks of life and adapted poorly to cardiac stressors [4]. The disruption of this pathway is believed to be the primary means of trastuzumab mediated cardiotoxicity however the entire mechanism has yet to be fully elucidated [5].
It is unknown if there is a correlation between degree of HER2 expression and degree of cardiotoxicity resulting from HER2 inhibition. Trastuzumab-associated cardiotoxicity was most commonly seen in patients receiving concurrent anthracyclinetrastuzumab based regimens. A phase III trial reported 27% of patients developed some grade of left ventricular dysfunction [3]. Due to the concern for development of congestive heart failure, cardiac monitoring guidelines were developed and subsequently adopted as standard of care for patients receiving this therapy. Additional risk factors have been identified for the development of cardiotoxicity including age, female sex, high dose radiation therapy, smoking, hypertension, diabetes, and dyslipidemia. The current trastuzumab monitoring recommendations indicate that LVEF monitoring should occur at baseline and at 3-month intervals during treatment [6]. Inconsistencies related to adherence to cardiac monitoring guidelines often occur in clinical practice due to an absence of data on if this rigorous monitoring is justified for all patients or if only certain populations necessitate increased monitoring [1,3,7].
There is no universal definition of cardiotoxicity. However, most clinical trials define cardiotoxicity by a serial decline in LVEF. The American Society of Echocardiography consensus document defines cardiotoxicity as LVEF drop ≥10% to a value of <53%. In the phase 3 Herceptin Adjuvant (HERA) trial, the cardiac review and evaluation committee defined cardiotoxicity as an asymptomatic reduction in LVEF ≥10% from baseline to below 50% or if patients experienced severe (New York Heart Association functional class III or IV) or symptomatic heart failure [8,9]. Additional trials and the trastuzumab package insert, have defined cardiotoxicity as a decrease in LVEF of ≥16% from baseline or LVEF below normal limits and ≥10% decrease from baseline. More recently myocardial strain has been proposed as a potential means of identifying subclinical myocardial injury and may help predict impending changes in LVEF [1,4,10-13]. A relative reduction of global peak systolic longitudinal strain of 10-11% at 3 or 6 months during trastuzumab treatment predicts future cardiotoxicity in women receiving trastuzumab for breast cancer. Additionally, the American Society of Echocardiography has included a decline in global peak systolic longitudinal strain of >15% during cancer treatment in their definition of cardiotoxicity. With no overtly clear definition of cardiotoxicity, clinicians may need to utilize a combination of expert guidance recommendations [14,15].
While it is known that trasuzumab can induce cardiotoxicity, the degree of HER2 expression and its correlation to the development of cardiotoxicity remains unknown. The degree of HER2 expression is an important factor in the decision to treat patients with trastuzumab. The American Society of Clinical Oncology/College of American Pathologists has released guideline recommendations for HER2 testing to improve its accuracy and reliability. These guidelines recommend at least one tumor sample be tested for either HER2 protein expression via immunohistochemistry (IHC) or HER2 gene expression via fluorescent in situ hybridization (FISH) reported as a ratio of HER2 gene signals relative to those of chromosome 17 (CEP17). A patient may qualify for treatment by meeting the minimum threshold requirement of HER2 gene expression (HER2:CEP17 ratio ≥2 or if HER2:CEP17 ratio <2 and ≥6 HER2 signals/cell), while other patients may far surpass the minimum expression required for treatment [15]. The purpose of our study was to identify and describe the relationship between degree of HER2 expression and incidence of cardiotoxicity in patients receiving trastuzumab therapy.
Methods
Patients receiving trastuzumab therapy at Froedtert & the Medical College of Wisconsin from May 1st, 2004 to July 1st, 2017 were evaluated after receiving IRB approval via retrospective chart review. Eligible patients carried a diagnosis of metastatic or nonmetastatic breast, metastatic gastric, or metastatic esophageal cancer. Patients included in the study had received at least one dose of trastuzumab, had a baseline LVEF measured prior to receiving trastuzumab therapy, at least one LVEF measurement for comparison while receiving trastuzumab, and a reported FISH HER2:CEP17 ratio. Measurement of LVEF could occur via multigated acquisition scan (MUGA) or echocardiogram (ECHO). Patients who were less than 18 years of age or had insufficient information in their medical record were excluded from the study. Multiple data points were collected for the analysis including patient demographic information, cardiac comorbidities, cancer diagnosis and stage, trastuzumab dose, dosing interval, dates of administration, number of doses received, FISH HER2:CEP17 ratio, and IHC result if available. Information regarding the method of LVEF measurement, LVEF value at baseline and while receiving trastuzumab therapy, global peak systolic longitudinal strain measurements, and number of trastuzumab doses prior to experiencing cardiac toxicity were also collected. Cardiac comorbidities as well as exposure to cardiotoxic therapies, such as previous exposure to radiation, anthracyclines, and other anti-HER2 therapies were also collected to adjust for confounding variables.
The primary objective of this study was to describe the relationship between HER2 receptor expression and cardiotoxicity in patients treated with trastuzumab. The secondary objectives were to identify the association between HER2 expression and absolute change in LVEF and time to cardiotoxicity in patients treated with trastuzumab. For the purposes of this study, cardiotoxicity was defined as a decrease in LVEF ≥ 16% from baseline or a LVEF <50% and ≥10% decrease from baseline. Change in global peak systolic longitudinal strain of ≥15%, grade ≥3 left ventricular systolic dysfunction as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events v 4.03 (NCI CTCAE), or treatment discontinuation due to trastuzumab cardiotoxicity as determined by physician was also used to meet criteria for primary end point of cardiotoxicity. Froedtert & the Medical College of Wisconsin IRB approved completion of this study.
Statistics
Study variables were summarized using the mean, median, standard deviation, and range for continuous variables and frequency and percentage for categorical variables. There were no missing values among analytic variables. The primary predictor, HER2 receptor expression, was evaluated as both a continuous variable and as categories grouped by the median observed value (< 6 and 6+) and approximate quantiles (< 3, [3-6), [6-9), and 9+). Patient demographics and baseline clinical characteristics were compared between HER2 groups (< 6 and 6+) using Wilcoxon rank-sum tests for continuous variables and Fisher’s exact tests for categorical variables. All analyses were stratified by cancer stage (metastatic vs. non-metastatic) due to inherent differences in and length of trastuzumab treatment and dosing.
The primary outcome, cardiotoxicity, was analyzed using survival analysis methods. The survival time was taken as the time from start of therapy to the first observed cardiotoxicity event. Patients were censored at the last known follow-up or death up to 18 months. Kaplan-Meier methods were used to plot the (unadjusted) survival distributions of each HER2 group (median and quantile groupings) and the log-rank test was used to compare them. Multivariable Cox proportional hazards regression were used to compare time to cardiotoxicity by HER2 expression adjusting for age at first dose, presence of any comorbid conditions, concurrent radiation, and anthracycline exposure; two models were produced to assess HER2 as a linear predictor and alternatively using the median categories. The secondary outcome, reduction in LVEF, was analyzed similarly using survival methods. The event was defined as the first reduction from baseline LVEF of at least 16% or at least 10% with resulting LVEF below 50%. All statistical analyses were performed using R version 3.3.4 (R Foundation for Statistical Computing, http://www.R-project.org). All p-values were 2-sided and p < 0.05 was considered statistically significant.
Results
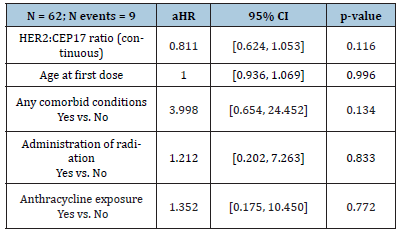
There were 44 (34 non-metastatic and 10 metastatic) patients with cardiotoxicity event(s) and 11 deaths without cardiotoxicity (all metastatic patients) within 18 months of follow-up; the median follow-up among patients without cardiotoxicity or death was 11.7months (range, 9.0-16.2 months). Overall one-year incidence of cardiotoxicity was 14.2% [10.7%, 18.9%], with incidences of 13.6% [9.9%, 18.8%] in non-metastatic patients and 16.7% [9.1%, 30.5%] in metastatic patients. The median survival time (time at which half of all patients are expected to experience cardiotoxicity) was not reached within 18 months. There was no significant difference in survival from cardiotoxicity between HER2:CEP17 groups (<3, 3-6, 6-9, 9+) among non-metastatic patients (log-rank test p = 0.72) or metastatic patients (log-rank test p = 0.17) (Figure 1). The HER2:CEP17 ratio (continuous) was not significantly associated cardiotoxicity in non-metastatic patients (aHR 1.00 [0.94, 1.07], p = 0.95) or metastatic patients (aHR 0.88 [0.72, 1.07], p = 0.19) after adjusting for baseline age, comorbidities, radiation exposure, and anthracycline exposure. In the multiple regression analysis of non-metastatic patients, anthracycline exposure (aHR 2.73 [1.28, 5.81], p = 0.009), baseline comorbidities (aHR 2.41 [1.14, 5.09], p = 0.021), and younger age at first dose (aHR 0.95 [0.92, 0.98], p = 0.002) were significantly associated with increased hazard of cardiotoxicity.
Figure 1:Survival from cardiotoxicity
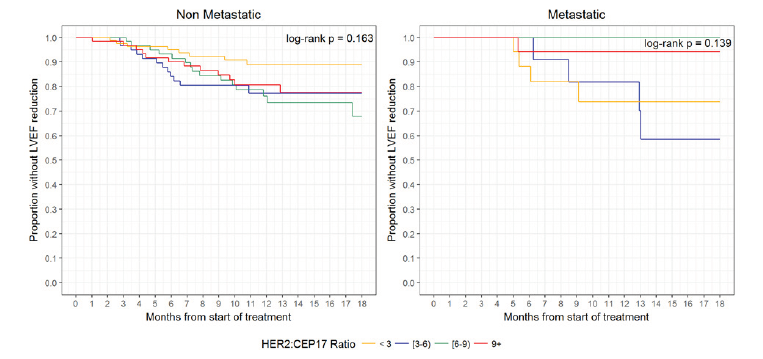
There were 56 (47 non-metastatic and 9 metastatic) patients with LVEF reduction and 12 deaths without cardiotoxicity (all metastatic patients) within 18 months of follow-up; the median follow-up among patients without LVEF reduction or death was 12.4 months (range, 9.6-16.8 months). Overall one-year incidence of LVEF reduction was 17.4% [13.5%, 22.3%], with incidences of 18.6% [14.2%, 24.4%] in non-metastatic patients and 12.3% [6.2%, 24.8%] in metastatic patients. The median time to LVEF reduction was not reached within 18 months. There was no significant difference in survival from LVEF reduction between HER2:CEP17 groups (<3, 3-6, 6-9, 9+) among non-metastatic patients (log-rank test p = 0.16) or metastatic patients (log-rank test p = 0.14) (Figure 2). The HER2:CEP17 ratio (continuous) was not significantly associated with LVEF reduction in non-metastatic patients (aHR 1.02 [0.97, 1.07], p = 0.53) or metastatic patients (aHR 0.81 [0.62, 1.05], p = 0.12) after adjusting for age, baseline comorbid conditions, radiation exposure, and anthracycline exposure (Tables 2 & 3). Only anthracycline exposure was significantly associated with increased hazard of LVEF reduction (aHR 2.37 [1.27, 4.43], p = 0.007) in non-metastatic patients.
Figure 2:Survival from LVEF reduction
Discussion
Our evaluation of this retrospective cohort of 324 patients with HER2 positive breast and gastric cancer, followed up to 13 years demonstrated no association between the HER2:CEP17 ratios and the incidence of cardiotoxicity. Furthermore, our study revealed that >90% of cardiotoxicity events occurred during the first year in both the non-metastatic and metastatic patient population. We therefore limited statistical analysis to 18 months. The rarity of events beyond 1 year of therapy in the non-metastatic setting supports a lack of long-term toxicity once therapy is complete. Within the metastatic setting, trastuzumab therapy was tolerated remarkably well and supports a lack of cumulative dose related toxicity which may allow for less frequent cardiac monitoring in metastatic patient receiving greater than one year of therapy. These data represent a real-world cohort of patients with pre-exiting cardiac comorbidities which adds to the external validity of our findings.
To our knowledge, this is the first investigation as to whether the degree of HER2 gene expression is related to patients’ risk for developing trastuzumab-induced cardiotoxicity. Clinical prediction tools have been published which attempt to identify patients at highest risk for cardiotoxicity in the setting of HER2 positive breast cancer treated with trastuzumab. Romond et al. [1] developed a prediction tool, which revealed age and baseline LVEF played the biggest role in cardiotoxicity development. A second prediction tool was developed in 2014 by Ezaz et al. [17] which revealed age, adjuvant chemotherapy, history of cardiac disease, and cardiac risk factors to be predictive of cardiotoxicity [16]. Currently, no prediction tool has incorporated biomarkers or diagnostic tests and imaging into their model [17]. It is important to note that our study utilizes HER2:CEP17 ratios from a patient’s tumor cells. Without matched cardiac tissue testing for HER2 expression it is not possible to ascertain whether there is a correlation between tumor cell expression of HER2 and cardiac myocyte expression. Therefore, it is possible HER2:CEP17 ratios directly from cardiac tissue could be predictive of trastuzumab related cardiotoxicity, however obtaining these samples without another compelling indication is largely impractical.
Cardiotoxicity is an important potential cause of treatment delay and early discontinuation of treatment which ultimately results in sub-optimal treatment of the patient’s malignancy. Despite longterm follow-up of patients treated with trastuzumab and years of research on the prediction of cardiotoxicity, there is currently no standardized model available to guide risk stratification for trastuzumab related cardiotoxicity in today’s clinical practice. With the continued use of anti-HER2 targeted therapies, as well as other anti-cancer agents with cardiotoxicity potential, further research is needed to identify patients who are at highest risk for therapy related toxicity. Our study captured a heterogeneous population of patient receiving trastuzumab, both those that are healthy and those that have comorbidities. Although it was retrospective single center study, we were able to follow our patients for up to thirteen years after receiving therapy which allowed optimal time to capture cardiotoxicity events.
Conclusion
In this retrospective study, tumor cell HER2:CEP17 ratio did not predict incidence or time to onset of cardiotoxicity associated with trastuzumab. With the growing number of patients living longer with cancer, it is important to assess a patient’s risk for cardiac dysfunction as a result of chemotherapy treatment. Further research is needed to find novel patient characteristics to create standardized risk stratification tools and monitoring guidelines for patients receiving trastuzumab in an attempt to achieve a high level of cardiac safety without sacrificing outcomes or incurring unnecessary medical expense and patient inconvenience.
Declarations
Ethics approval and consent to participate. This study was approved by the Froedtert & the Medical College of Wisconsin IRB.
Availability of Data and Materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Author Contributions
CL was principal investigator of the study and lead author of the manuscript. CO served in an advisory role in study design and implementation with expertise in trastuzumab use in GI malignancy. She provided guidance in data analyzation as well as key edits and discussion points to the manuscript.
EW served in an advisory role in study design and implementation with expertise in trastuzumab use in breast cancer. She provided guidance in data analyzation as well as key edits and discussion points to the manuscript. AU served in an advisory role in study design and implementation with expertise in clinical trials. BA significantly contributed to data collection as well as authored the methods portion of the manuscript. LR provided statistical analysis and guidance in study design as well as authored the statistics and results section of the manuscript. SK served in an advisory role with expertise in the treatment of breast cancer. As our physician champion she provided key guidance in study design, manuscript edits and discussion contributions.
References
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16): 1673-1684.
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, Procter M, Suter TM, et al. (2013) Herceptin adjuvant (HERA) trial study team: 2 years versus 1 year of adjuvant trastuzumab for HER2-poositive breast cancer (HERA): An open-label, randomized controlled trial. Lancet 382(9897): 1021-1028.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783-792.
- Seidman A, Hudis C, Pierri MK, Shak S, Paton V, et al. (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20(5): 1215-1221.
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, et al. (1998) Neuregulin’s promote survival and growth of cardiac myocytes: Persistence of ERbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273: 10261-10269.
- G Curigliano, D Cardinale, T Suter, G Plataniotis, E de Azambuja, et al. (2012) Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Annals of Oncology 23(7): vii155-vii166.
- Tan-Chiu E, Yothers G, Romond E, Geyer CE, Ewer M, et al. (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer. J Clin Oncol 23(31): 7811-7819.
- Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, et al. (2016) Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: Getting to the heart of the matter. J Clin Oncol 34(10): 1030-1033.
- Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, et al. (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 27(9): 911-939.
- Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, et al. (2002) Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A 99(13): 8880-8885.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, et al. (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354: 809-820.
- Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, et al. (2011) Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 29(34): 4491-4497.
- Herceptin®(trastuzumab) (2017) Genentech Inc South, San Francisco, USA.
- Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, et al. (2013) Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 26(5): 493-498.
- Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, et al. (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline update. J Clin Oncol 31(31): 3997-4013.
- Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, et al. (2012) Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized control trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol 30(31): 3792-3799.
- Ezaz G, Long JB, Gross CP, Chen J (2014) Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab for breast cancer. J Am Heart Assoc 3(1): e000472.
© 2019 Cole Lightfoot. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






