- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Vagally Mediated Bradycardia and Increased Vagal Tone: Interest of Cardiovascular Autonomic Reflexes Testing
Romani I*, Soukrat S and El Hattaoui M
Department of Cardiology, Morocco
*Corresponding author: Romani I, Department of Cardiology, Morocco
Submission: February 13, 2019Published: March 13, 2019

ISSN 2578-0204Volume2 Issue5
Abstract
Introduction: The autonomic nervous system (ANS) controls all body functions. Dysregulation of this system may be responsible of bradycardia. The main objective of our study is to describe the autonomic profile of patients with bradycardia and to determine, through testing cardiovascular autonomic reflexes its involvement in the pathogenesis of idiopathic symptomatic bradycardia.
Material and methods: This are a retrospective study including 72 patients with bradycardia. The study was conducted in the ANS unit of the cardiology department in Mohamed VI hospital of Marrakech. All patients had clinical examination, electrocardiogram, biological exam and autonomic nervous system tests.
Results: The average age of the patients was 44 years old with a female predominance. The mean heart rate was 50bpm. Patients had polymorphic functional symptoms. The autonomic profile of patients with bradycardia revealed a significant predominance of vagal hyperactivity, which is present in 83.3% of patients. Increased vagal tone was associated with a peripheral sympathetic alpha deficiency in 30,5% of the cases, a peripheral sympathetic beta deficiency in 25% and even a peripheral sympathetic beta hyperactivity in 54,1% of patients. The main autonomic syndromes found were: postural orthostatic tachycardia syndrome (POTS) in 34,7% of patients, orthostatic hypotension in 25% of the cases, vasovagal syncope in 8,3%, and the baro-receptor abnormality in 12,5% of the patients. The treatment, depending on the autonomic abnormality showed an improvement in the functional state of the patients.
Conclusion: Autonomic profile study helps to explain the causes of symptoms described by the patients with bradycardia whose clinical and conventional paraclinical examinations are normal.
Keywords: Bradycardia; Autonomic nervous system; Increased vagal tone; Autonomic profile
Abbreviations: ANS: Autonomic Nervous System; SNS: Sympathetic Nervous System; PNS: Parasympathetic Nervous System; HR: Heart Rate; DB: Deep Breathing; BP: Blood Pressure; HG: Hand Grip; MS: Mental Stress; OT: Orthostatic test
Introduction
The autonomic nervous system (ANS) controls all body functions and maintains internal homeostasis and stress responses. The ANS is divided into two parts: the sympathetic (SNS) and parasympathetic (PNS) nervous system. The two components are too often considered to be antagonistic. While their actions on various organs may appear to be in opposition, the two systems are, in fact, complementary [1]. The ANS is a major regulator of the cardiovascular system. It regulates heart rate (HR) and blood pressure in the short-term to cope with everyday situations. Parasympathetic (vagal) modulation decreases the HR and cardiac contractility, whereas activity of the sympathetic branch opposes these effects and regulates peripheral vasoconstriction [2]. Bradycardia is a slowing of the heart rate below normal with a heart rate of less than 60 beats per minute in the adult and less than 80 beats per minute in the infant. Bradycardia may occur due to many cardiac and non-cardiac factors. Dysregulation of ANS, called dysautonomia, may be responsible of bradycardia. The main objective of our study is to describe the autonomic profile of patients with sinus bradycardia and to determine, through testing cardiovascular autonomic reflexes its involvement in the etiopathogenesis of idiopathic symptomatic bradycardia.
Material and Methods
Selection criteria
This is a retrospective study conducted from January 2017 to June 2018 that included 72 patients with bradycardia. The study was conducted in the ANS unit of the cardiology department in Mohamed VI hospital of Marrakech. The study of cardiovascular autonomic reflexes was intended to confirm the dysautonomia nature of bradycardia.
Inclusion criteria: All patients with heart rate < 60BPM. These patients, who have arrived in ANS unit exploration, usually have normal clinical and para-clinical examinations (biology, radiology and electroencephalogram) and any obvious cause of bradycardia have been eliminated.
Exclusion criteria: Other causes of bradycardia (atrial fibrillation, hypothyroidism, cardiopathy) were excluded from this study.
Description of the tests
The tests were done while all patients were fasting, and after cessation of any treatment for at least 48 hours. The patient was initially placed in calm. Monitoring of blood pressure (BP) was performed using a Dynamap and HR by means of a display screen. Basic BP and HR were measured at rest, every five minutes for at least 30 minutes. We have proceeded then to the different tests, interspersed with periods of rest, as described by Ewing [3,4], detailed by Phillip Low [5].
The Deep breathing test (DB): This test analyzes the vagal response. The result is expressed as a percentage: (Maximum RRMinimum RR)/Min RR. The normal result is 30%. It depends on the age of the subject; it is generally higher with young people [6]. The Isometric contraction or Hand Grip test (HG): During, three minutes the patient performs a manual pressure of 50% of the maximum with assistance of a dynamometer. The muscular contraction involves a rise in BP related to an increase of sympathetic nerve activity at the muscular level that is effort dependent and time-dependent [7]. The peripheral alpha sympathetic nerve response is given by the increase of the BP.
The Mental Stress test (MS): The patient performs mental arithmetic calculations by removing the number 7 successively from 200. The result is an increase in BP and in HR by activation of the central sympathetic nerve [8]. In mental stress, the central sympathetic nerves activities “α” was evaluated by measuring the variations of BP as bellow [8,9].
The Tilt test, Orthostatic test OT: It allows the study of the parameters (BP and HR) in orthostatism, for ten minutes, compared to the decubitus values. This test was not available at out unit. It was replaced by stand-up test which consists of measuring changes in HR and BP in response to an active stand up during 5 to 45 minutes. The test we performed in our patients lasts an average of 10 minutes. A decrease in systolic BP of 20mmHg and 10mmHg diastolic BP below 94mmHg, maintained for at least minus five minutes is considered hypotension orthostatic [7,10,11]. An increase in heart rate of 30 beats or more for three minutes is considered an idiopathic postural tachycardia syndrome. (POTS) The vagal response is also evaluated in the primary orthostatic 30 seconds. An increase of 10% than the basic HR is considered normal, above of 10%, we talk about vagal hyperactivity, below 10% is vagal disability [5,7]. Each autonomic test gives rise to a measurement response to stimulation in relation to the baseline state. Results are expressed as a percentage for all the stimuli both sympathetic and vagal. For sympathetic stimulations relating to the measurement of the variation in BP, only the systolic BP values were analyzed.
Statistical analysis
Descriptive statistics include ranks, mean and standard deviation for quantitative variables and frequency and percentage for qualitative variables. All statistical analyzes were performed using the SPSS 10 software.
Result
Figure 1:
The mean age of the population was 44±14.4 years (range 17 years to 70 years), with 66% of women. The mean baseline HR was 50±7,6bpm. The mean baseline systolic BP (SBP) was 112,3±16, 3mmHg and the average diastolic BP (DBP) was 65,4±9mmHg. Patients had polymorphic functional symptoms. The functional signs of dysautonomia are mainly dominated by orthostatic intolerance (91,6% of patients), cardiovascular disorders (88.8% of patients), neurological disorders (87,5% of patients) and gastrointestinal disorders (70.8% of patients). Signs occurred mostly on orthostatism or effort. The delay of symptom progression exceeded 6 months in all our patients (Table 1). Cardiovascular autonomic tests demonstrate the autonomic dysfunction in 87% of patients. The increased vagal tone is mostly found in 83,3% of patients in particular at DB test (Figure 1). The mean vagal response to DB is 57, 1±11,4 (range 39% to 99%). The disturbance of the vagal response to DB is found in 87.4% of patients with hyperactivity in 83,3% of patients and deficiency in 4,1% of patients (Table 2). In 66% of cases, patients had very high vagal response exceeding 65%. This severe vagal hyperactivity is dominant in patients under 35 years of age. The mean vagal response to HG is 27±4, 7 (range 25% to 71%). The vagal hyperactivity response to HG was found in 62, 5 % of patients whereas 18% of patients had vagal deficiency.
The mean vagal response to OT is 25±5,8 (range 19 to 48%). The mean peripheral sympathetic response alpha (alpha SP) to HG was 13,5±3,1% and to OT 14,6±3,7. The disruption of the alpha SP response to HG was found in 47,1% of patients with deficiency in 30,5% of patients and hyperactivity in 16,6 % of patients (Table 3). The central sympathetic response alpha (alpha SC) was increased in 29,1% of patients while it was deficient in 40,1% of patients. The disturbance of the central sympathetic response beta (beta SC) to MS was found in 83,3 % of patients with deficiency in 50% of patients and hyperactivity in 33,3% of patients (Table 4).
Table 1:Functional symptoms of patients.

Table 2:Vagal response.
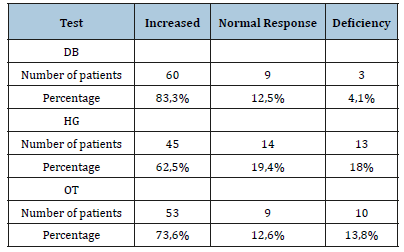
Table 3:Peripheral sympathetic response alpha.
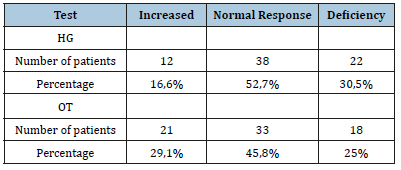
Table 4:Alpha SC et Beta SC response to MS.
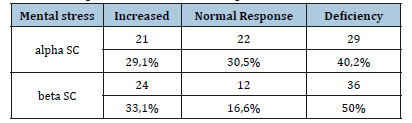
Table 5:Beta peripheral sympathetic response.

Increased peripheral sympathetic response beta was found in 39 patients (54, 1%) (Table 5), whom 34,7% had idiopathic postural tachycardia syndrome (POTS syndrome). Indeed, besides sympathetic and parasympathetic abnormalities, we have been able to individualize other syndromes like POTS syndrome in 25 patients (34,7%), orthostatic hypotension in 18 patients (25%), vasovagal syncope in 6 patients (8,3%), and the baro-receptor abnormality in 9 patients (12,5%). The treatment depending on the autonomic abnormality (hygienodietetic measures, ethylephrine, fludrocortisone, Phenobarbital, serotonin recapture inhibitor) showed an improvement in the functional symptoms after one month of treatment in 94% of patients. The treatment was conducted as monotherapy (60% of patients), or in combination therapy depending on the severity of the symptoms. However, hygienodietetic measures were sufficient in 40% of cases.
Discussion
Bradycardia may occur due to a decrease in the frequency of impulse propagation within the heart and/or impaired electrical intracardiac conduction of that impulse once propagated. These events can occur because of primary heart disease affecting the conduction system or as a secondary effect of more generalized macro cardiac disease (chronic ischemic heart disease, valvopathy and myopathy). Additionally, extra cardiac factors can act upon a normal or a diseased heart yielding bradycardia as an appropriate response to those abnormal extracardiac factors. Vagally-mediated bradycardia would be the most common such example. The autonomic nervous system controls all body functions. Balanced cardiac ANS function is based on strong parasympathetic and efficient, but not overactive, sympathetic modulation of the heart. Dysautonomia may be responsible of bradycardia. It is important to know if bradycardia is symptomatic and whether or not it is related to a dysfunction of the ANS because a large number of symptomatic brady cardia remains without etiology and the implication of the ANS is to be considered. This was the aim of our study. Through the evaluation of the 72 patients, we were able to identify certain abnormalities and show the presence of dystaunomia in the autonomic profile of 87% of patients. In our study, a majority of women were found to be affected (66% of patients). As described in the literature, the women are more concerned with autonomic disorders than man [10]. Our patients had very polymorphic symptoms. This was confirmed by El Honsalis I [8] study where the included patients had a rich and various symptomatology. These signs are related to systemic hypo perfusion. They are frequently not tolerated and increased by the prolonged standing, confined atmosphere, on postprandial, after exertion, emotion or pain [11]. In our series, functional symptoms are dominated by orthostatic intolerance, with chronic fatigue and migraine. Indeed, it should be noted that the increased vagal tone with sympathetic deficiency would be the main principle of the pathogenesis of migraine [9,12].
The autonomic profile of patients with bradycardia revealed a significant predominance of vagal hyperactivity, which is present in 83.3% of patients. Vagal hyperactivity was associated with a peripheral sympathetic alpha deficiency in 30, 5% of cases, and even a peripheral sympathetic beta deficiency in 25% of patients. The prevalence of increased vagal tone dysautonomia is not well determined since it is an abnormality in full expansion and still little known to the medical team. The lack of the bibliographic references was very important at the beginning of our research. In our modest series, there is a clear predominance of severe vagal hyperactivity in 66% of patients, this severe enhanced vagal activity is dominant in all patients under 35 years of age. The young patients represent 40% of the patients explored. This confirms the data of the literature since the vagal hyperactivity with sympathetic deficiency is a frequent autonomic dysfunction in the young subject, contrary to the sympathetic hyperactivity which is the privilege of the elderly subject especially diabetic and hypertensive [13-15].
In our study, increased vagal tone was associated with peripheral sympathetic beta hyperactivity in 54,1% of patients. Although surprising, in 34,7% of patients with baseline bradycardia, the ANS study objectified POTS syndrome. POTS or idiopathic orthostatic tachycardia is a form of orthostatic intolerance that affects mostly women. It is a badly known pathology because its diagnosis is not based on the conventional methods of investigation. The orthostatic test allows to make the diagnosis easily. It is defined according to Phillip Low [5] by an increase of the HR higher than 30bpm compared to the base HR, during the passage to the standing station. This condition is characterized by a central hypovolemia, a high blood flow in the lower limbs to standing with a sympathetic abnormality [16-18]. Orthostatic hypotension syndrome found in 25% of patients is widely developed in the literature. According to Grubb et Blanc [11], it is related to a dysfunction of the ANS. It is defined by a reduction of more than 15% of the systolic pressure during the transition from the supine to the upright position. The vasovagal syncope has been found in 8,3% of patients. It associates hypotension with paradoxical bradycardia occurring in orthostatism [19]. The patients who have benefited from the ANS study are satisfied because they have a diagnostic, the cause of their suffering is revealed and above all the treatment is available and significantly improves the functional Symptoms.
Conclusion
There is often a symptomatic bradycardia of which no cause is detectable. Autonomic profile study helps to explain the causes of symptoms described by the patients with bradycardia whose clinical and conventional paraclinical examinations are normal. This was confirmed by our study that had shown the existence of autonomic dysfunction in nearly 87% of our patients. It appears that this symptomatic idiopathic bradycardia is secondary to increased vagal tone with sympathetic deficiency.
References
- Goldstein DS (2002) Dysautonomias: Clinical disorders of autonomic nervous system. Ann Intern Med 137: 753-763.
- Goldstein DS (1997) Sympathetic cardio neuropathy in dysautonomia. N Engl J Med 336(10): 696-702.
- Ewing DJ, Irving JB, Kerr R, Wildsmith JA, Clarke BF (1974) Cardiovascular responses to sustained handgrip in normal subjects and in patients with diabetes mellitus: A test of autonomic function. Clin Sci Med 46(5): 295- 306.
- Ewing DJ, Martyn CN, Young RJ, Clarke BF (1985) The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8: 491-498.
- Low PA (1997) Laboratory evaluation of autonomic function. In: Low PA (Ed.), Clinical autonomic disorders (2nd edn), Lippincott-Raven Publishers, Philadelphia, Pennsylvania, USA, pp. 179-208.
- Johansen TL, Kambskar G, Mehlsen J (1997) Heart variability in evaluation of the autonomic nervous system. Ugeskr laeger 159(45): 6666-6671.
- Coghlan HC (1996) Orthostatic intolerance: Mitral valve prolapse. In: Robertson D, Low PA, Polinsky J (Eds.), Primer on the autonomic nervous system. Academic Press, San Diego, California, USA, pp. 283-328.
- Elhonsali I, Benjelloun H, Coghlan L, Benomar M (2004) Symptomatologie fonctionnelle cardiovasculaire: Intérêt de l’étude du profile autonomique. Annales de Cardiologie et d Angéiologie 53(3): 137-143.
- Edvinsson L (2001) Aspects on the pathophysiology of migraine and cluster headache. Pharmacol Toxicol 89(2): 65-73.
- Mathias CJ, Bannister R (1999) Autonomic failure. A text book of clinical disorders of the autonomic nervous system (5th edn).
- Grubb BP, Blanc JJ (1999) Hypotension orthostatique due à un dysfonctionnement du systèm nerveux autonomy. Arch Mal Caeur 92: 43-51.
- Benjelloun H, Birouk N, Slaoui I, Coghlan L, Bencheikh BOA, et al. (2005) Profile autonomique des patients migraineux. Neurophysiologie Clinique 35(4): 127-134.
- Low PA (1984) Laboratory evaluation of autonomic function. In: Dyck PJ (Ed.), Peripheral neuropathy, WB Saunders, Philadelphia, Pennsylvania, USA, p. 1139.
- Aboudrara S, Benjelloun H, Benazzouz A, Bendahmanne S, Coghlan L, et al. (2007) Evaluation de activate vagale par le test de la respiration profonde (Deep-Breathing). Neurophysiologie Clinique/Clinical Neurophysiology 37(1): 41-46.
- Benyamna I, Benjelloun H, Coghlan L, Benomar M (2010) Clinical symptoms in dysautonomia characterized by a decreased sympathetic tone with enhanced vagal activity. Clinical Autonomic Research 16(2).
- Jacob G, Biaggioni I (1999) Idiopathic orthostatic intolerance and postural tachycardia syndrome. Am J Med Sci 317(2): 88-101.
- Grubb BP, Blanc JJ (2000) Tachycardie orthostatique idiopathique: Etiologie, diagnostic et traitement. Arch Mal Caeur 93(1): 79-85.
- Low PA, Schondorf R, Novak V, Sandroni P, Gehrking TL, et al. (1997) Postural tachycardia syndrome. In: Low PA (Ed.), Clinical Autonomic Disorders (2nd edn), Lippincott-raven publishers, Philadelphia, Pennsylvania, USA, pp. 681-697.
- Sandroni P, Gehrking TL, Benmermch EE, Shen WK, Low PA (1996) Certain cardiovascular indices predict syncope in the postural tachycardia syndrome. Clinical Autonomic Research 6(4): 225-231.
© 2019 Romani I. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






