- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Evaluation of Cardiac Biomarkers after Treatment with the Biofield Treated Test Item in Cardiomyocytes Cell Line (H9c2)
Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd, Bhopal, Madhya Pradesh, India
Submission: January 08, 2019;Published: January 18, 2019

ISSN 2578-0204Volume2 Issue5
Abstract
The study objective was to evaluate the cardioprotective activity of Biofield Energized test item (DMEM) in rat cardiomyocytes (H9c2) cells. The test item (DMEM medium) was divided into three parts, first part received one-time Consciousness Energy Healing Treatment by a renowned Biofield Energy Healer, Dahryn Trivedi and was labeled as the one-time Biofield Energy Treated (BT-I) DMEM, while second part received the two-times Biofield Energy Treatment and is denoted as BT-II DMEM. The third part did not receive any treatment and defined as the untreated DMEM group. MTT cell viability assay of the test sample showed more than 95% viable cells in the BT-I and BT-II groups, respectively suggested a nontoxic nature of the test substance. The BT-II group showed 16.28% restoration of cytoprotective function compared to the t-BHP induced group. The level of lactate dehydrogenase (LDH) was significantly inhibited by 48.36% and 47.89% in the BT-I and BT-II groups, respectively compared to t-BHP induced group. Level of creatine kinasemyocardial band (CK-MB) was significantly suppressed by 59.5% in the BT-I and BT-II groups compared to the t-BHP induced group. Reactive oxygen species (ROS) level was significantly suppressed by 120.08% and 116.20% in the BT-I and BT-II groups, respectively. Besides, BT-I and BT-II groups showed 33% and 48.5% inhibition of apoptotic cells, respectively compared to the t-BHP induced group. Altogether, result suggested that Biofield Treated test items significantly improved different cardiac parameters. Thus, Consciousness Energy Healing (The Trivedi Effect®) Treatment could be utilized as a cardio-protectant against several cardiac disorders such as heart failure, coronary artery disease, heart attack, congenital heart disease, cardiomyopathy, arrhythmias, etc.
Keywords: The Trivedi Effect®; Consciousness energy healing; H9c2; Heart health; CK-MB; ROS; LDH; Apoptosis; Reactive oxygen species; Tert-Butyl hydroperoxide
Abbreviations:CAM: Complementary and Alternative Medicine; BT-I: One-Time Biofield Energy Treatment; BT-II: Two-Time Biofield Energy Treatment; DMEM: Dulbecco’s Modified Eagle Medium; FBS: Fetal Bovine Serum
Introduction
Three main criteria to keep a healthy heart like opening blood vessels, strengthening the heart muscle, and controlling free radical damage by antioxidants. Cardiovascular disorders (CVDs) are the leading cause of morbidity and mortality in the developed world. Heart disorders are the major concern of population health worldwide. About 6 lakh peoples die due to heart disease in the United States every year; i.e., 1/4 deaths [1]. According to WHO estimates, in 2002, 16.7million people around the globe die due to cardiovascular diseases each year [2,3]. For the assessment of cardioprotective activity of a test compound cardiomyocytes were used as test system most of the cases [4]. Cardiomyocyte cells (H9c2) obtained from rats have been extensively utilized as a test system as an alternative to human cardiomyocytes model [5]. Moreover, in vitro experiment has some advantages over in vivo study like need of few animals, test material, and provides high accuracy data [6]. Numerous experimental findings suggested that the effect of Energy Therapy in cancer patients through therapeutic touch [7], massage therapy [8], etc. Complementary and Alternative Medicine (CAM) therapies are preferred model of treatment, among which Biofield Therapy (or Healing Modalities) is one approach to enhance physical, emotional, mental, and human wellness. The National Center of Complementary and Integrative Health (NCCIH) has recognized and allowed Biofield Energy Healing as a CAM approach in addition to other therapies and medicines such as natural products, chiropractic/osteopathic manipulation, yoga, Tai Chi, massage, meditation, relaxation techniques, special diets, deep breathing, homeopathy, progressive relaxation, mindfulness, Qi Gong, Reiki, hypnotherapy, guided imagery, healing touch, acupuncture, movement therapy, pilates, traditional Chinese herbs and medicines, Ayurvedic medicine, rolfing structural integration, naturopathy, essential oils, aromatherapy, acupressure, and cranial sacral therapy. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [9]. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles [10]. This energy can be harnessed and transmitted by the experts into living and non-living things via the process of Biofield Energy Healing. The Trivedi Effect®- Consciousness Energy Healing Treatment has been reported with a significant revolution in the field of cancer research [11,12], materials science [13-15], microbiology [16-18], agriculture [19,20], nutraceuticals [21,22], and biotechnology [23,24]. Besides, The Trivedi Effect® also significantly improved bioavailability of various low bioavailable compounds [25-27], an improved overall skin health [28,29], bone health [30-32], human health and wellness. Based on the excellent contribution of Biofield Energy in wide spectrum of areas, authors intend to extend the treatment modality to study the impact of the Biofield Energy Healing Treatment (The Trivedi Effect®) on the test item (DMEM) for cardiomyocytes cell line (H9c2).
Materials and Methods
Chemicals and reagents
N-acetyl cysteine (NAC), 2′,7′-Dichlorofluorescin diacetate (DCFDA), 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical Co. (St. Louis, MO). Antibiotics solution (penicillin-streptomycin) was purchased from HiMedia, India. Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco, India. Creatine kinase- MB (CK-MB) and lactate dehydrogenase (LDH) kits were obtained from Biovision, USA. Annexin-V kit was purchase from Guava Technologies, USA. The positive control, trimetazidine (TMZ) was procured from Zliesher Nobel, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Biofield energy healing strategy
The test item (DMEM) was used in this experiment was divided into three parts, and one portion was considered as the untreated DMEM group, where no Biofield Treatment was provided. Further, the untreated DMEM group was treated with “sham” healer for comparison purpose. The “sham” healer did not have any knowledge about the Biofield Energy Healing Treatment. The second portion of the test item was received one-time Biofield Energy Treatment and referred as the BT-I group and the third part was given two-times Biofield Energy Treatment and defined as the BT-II group. Both the test items (BT-I and BT-II) were received Biofield Energy Healing Treatment (known as The Trivedi Effect®) under laboratory conditions for ~5 minutes through Dahryn Trivedi’s unique Biofield Energy Transmission process. Biofield Energy Healer was located in the USA; however, the test items were located in the research laboratory of Dabur Research Foundation, New Delhi, India. Biofield Energy Healer in this experiment did not visit the laboratory, nor had any contact with the test samples. After that, the Biofield Energy Treated and untreated test items were kept in similar sealed conditions and used for the study as per the study plan.
Assessment of cell viability using MTT assay
The cell viability was performed by MTT assay in H9c2 cell line. The cells were counted and plated in a 96-well plate at the density corresponding to 10X103 cells/well/180μL in DMEM+10% FBS. The cells in the above plate(s) were incubated for 24hours in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. Following incubation, the medium was removed, and the following treatments were given. In the test item group, 200μL of the test item was added to wells. Besides, in the positive control group, added 180μL of SFM with 20μL of positive controls were added from the respective 10X stock solutions. After incubation for 48hours, the effect of test item on cell viability was assessed by MTT assay. 20μL of 5mg/mL of MTT was added to all the wells and incubated at 37°C for 3hours. The supernatant was aspirated and 150μL of DMSO was added to all wells to dissolve formazan crystals. The optical density (OD) of each well was read at 540nm using Biotek Reader.
Effect of the test items on viability of H9c2 cells was determined using Equation (1):
% Cell viability = (100-% Cytotoxicity) (1)
Where, % Cytotoxicity = {(O.D. of untreated cells – O.D. of cells treated with test item)/ OD of untreated cells} *100
The concentrations resulting in ≥70% cell viability was taken as safe/non-cytotoxic for cytokine estimation.
Evaluation of cytoprotective effect of the test item
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted on a hemocytometer and seeded at a density of 5 X 103 cells/well/180μL in DMEM+10% FBS in a 96-well plate. Cells were incubated in a CO2 incubator for 24hours at 37°C, 5% CO2, and 95% humidity. After 24hours, the medium was removed, and the following treatments were given. In the test item group, 180μL of the test item was added to wells. In the positive control group, 160μL of SFM and 20μL of positive control from the respective 10X stock solution was added to wells. After 24hours of treatment, cells were treated with t-BHP at a final concentration of 250μM (20μL from the respective 10X stock) for 4hours. After 4hours, the protective effect of the test item on cell viability was assessed by MTT assay. The protective effect of the test item on survival of H9c2 cells against t-BHP induced damage was determined using Equation (2)
[(A-B)/(C-B)] *100 (2)
Where, A = O.D. of test item/positive control + t-BHP treated cells
B= O.D. of cells (t-BHP alone)
C = O.D. of untreated cells
Estimation of lactate dehydrogenase (LDH)
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted (using hemocytometer) and seeded (at a density of 0.12 X 106 cells/well/500μL) in DMEM+10% FBS in 48-well plates. Cells were incubated in a CO2 incubator for 24hours at 37°C, 5% CO2 and 95% humidity. After 24 hours, medium was removed, and following treatments were given. Test items (BTI and BT-II) groups (450μL of Biofield Treated DMEM), positive controls (trimetazidine and N-acetyl cysteine) groups (400μL of SFM), and (untreated DMEM) group (500μL of SFM) were added to the respective wells and incubate for 24hours. After that, cells were treated with 300μM of t-BHP (50μL from the respective 10X stock) for 2.5 hours. Supernatants were collected from each well and stored at -20°C till analyzed. Estimation of LDH in culture supernatants was done using Lactate Dehydrogenase Activity Colorimetric Assay Kit as per manufacturer’s instructions. LDH activity (nMoles/min/mL) was determined and the protective effect of test item was calculated using Equation (3):
[(A-B)/(A-C)] *100 (3)
Where, A = LDH activity in cells (t-BHP alone)
B= LDH activity in test items/positive controls + t-BHP induced cells
C = LDH activity in untreated cells
Estimation of CK-MB
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted on a hemocytometer. Cells were seeded at a density of 0.12 X 106 cells/well/500μL in DMEM+10% FBS in 48-well plates. Cells were incubated in a CO2 incubator for 24 hours at 37°C, 5% CO2 and 95% humidity. After 24hours, medium was removed, and treatments were given.
Test items (BT-I and BT-II) groups (450 μL of Biofield Treated DMEM), positive control (N-acetyl cysteine) group (400μL of SFM), t-BHP per se group (450μL of SFM), and negative control (untreated) group (500μL of SFM) were added to the respective wells and incubate for 24hours. After incubation for 24hours, cells were treated with 300μM of t-BHP (50μL from the respective 10X stock) for 2.5hours. Supernatants were collected from each well and stored at -20°C till analyzed. Estimation of CK-MB in culture supernatants was done using Creatine Kinase Activity Colorimetric Assay Kit as per manufacturer’s instructions. CK-MB activity (nMoles/min/mL) was determined and protective effect of test item on CK-MB activity was calculated using Equation (4):
[(A-B)/(A-C)] *100 (4)
Where, A = CK-MB activity in cells (t-BHP alone)
B= CK-MB activity in test items/positive controls + t-BHP treated cells
C = CK-MB activity in untreated cells
Assessment of reactive oxygen species (ROS)
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Then, the cells were counted with the help of a hemocytometer and seeded (at a density of 20X103 cells/ well/180μL in DMEM +10%FBS) in 96-well plates. Cells were incubated in a CO2 incubator for 24hours at 37 °C, 5 % CO2 and 95% humidity. Then, medium was removed, and treatments were given. About 180μL of the test item (TI), 160μL of SFM, 180μL of SFM, and 200μL of SFM was added to wells of test items, positive controls, t-BHP per se, and untreated DMEM groups, respectively and incubate for 24hours. After incubation for 24hours, cells were stained with DCFDA and washed the wells once with Hank’s Balanced Salt Solution (HBSS)+2%FBS solution and 180μL of SFM was added to each well. Protective effect of TI on ROS activity was calculated using Equation (5):
[(A-B)/(A-C)] *100 (5)
Where, A = Mean FU in Control cells (t-BHP alone)
B= Mean FU in TI/positive control + t-BHP treated cells
C = Mean FU in untreated cells
Effect of test item on apoptosis
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted using hemocytometer and seeded at a density of 0.25million/well/1mL in DMEM+10% FBS in 96- well plates. Further, the cells were incubated in a CO2 incubator for 24 hours at 37°C, 5 % CO2 and 95% humidity. After 24 hours, medium was removed, and the following treatments were given. The TI group received 900μL of Tis (BT-I and BT-II), positive control (N-acetyl cysteine) group received 800μL of SFM, t-BHP group received 900μL of SFM, and the untreated DMEM group provided 1mL of SFM to the corresponding wells and incubate for 24hours. After that, cells were treated with 300μM of t-BHP (100μL from the respective 10X stock) for 2.5hours. Then, the cells were stained with Annexin reagent for apoptotic population as follows: cells were gently harvested by trypsinization into prelabeled centrifuge tubes followed by pelleted and resuspended in 200μL of SFM. At 100μL of cell suspension was stained with 100μL of Annexin reagent for 30minutes in a dark condition at room temperature. Cells were acquired at flow cytometer (Guava technologies). The protective effect of the TI was calculated using Equation (6):
[(A-B)/(A-C)] *100 (6)
Where, A = % Apoptotic population in t-BHP
B = % Apoptotic population in test items/positive control + t-BHP treated cells
C = % Apoptotic population in untreated cells
Statistical analysis
All the values were represented as Mean± SEM (standard error of mean) of three independent experiments. The statistical analysis was performed using SigmaPlot statistical software (v11.0). For two groups comparison student’s t-test was used. For multiple group comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
Result and Discussion
MTT assay
Figure 1:
Effect of the test samples and positive controls on cell viability in H9c2 cells after 24 hours of treatment.
TMZ: Trimetazidine; NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
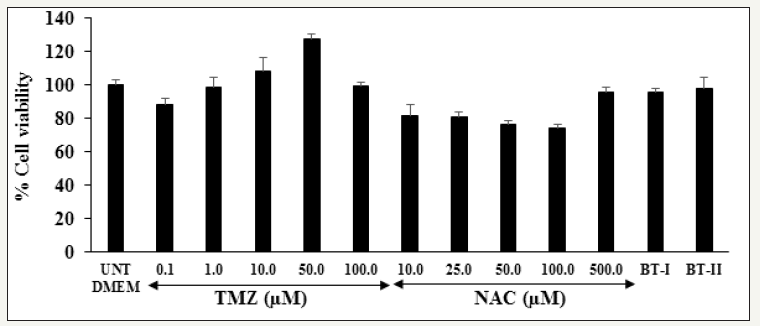
Figure 1 represented the percent cell viability of the positive controls and test items in H9c2 cells. Trimetazidine (TMZ), the reference standard was used in this experiment showed greater than 88% cell viability at the concentrations between 0.1 to 100μg/ mL, and N-acetyl cysteine (NAC) showed more than 74% cell viability upto 500μg/mL. The Biofield Energy Treated test items, BT-I (one-time Biofield Energy Treated DMEM) and BT-II (twotimes Biofield Energy Treated DMEM) showed 95.77% and 97.70% cell viability, respectively (Figure 1). The cell viability data indicated a safe and nontoxic profile of the test substances and further used in this experiment for the assessment of various cardiac parameters.
Evaluation of cytoprotective effect of the test item
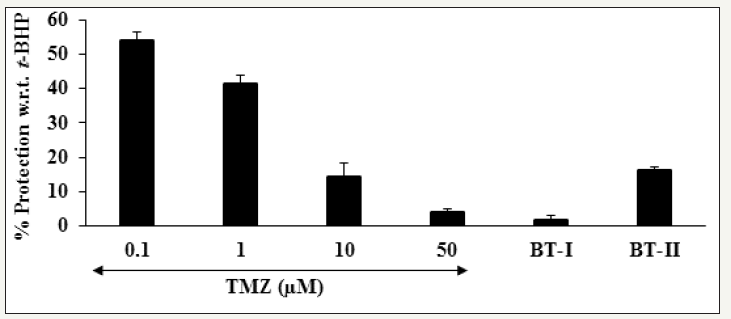
Evaluation of hepatoprotective activity of any test substances, tert-butyl hydroperoxide (t-BHP) is a well-recognized oxidative stress inducing agent in the in vitro cell-based assays [33,34]. The cytoprotective activity of the Biofield Treated test items on the restoration of cell viability in H9c2 cells was determined against t-BHP induced cell damage is shown in Figure 2. The positive control, trimetazidine (TMZ) showed 54.1%, 41.3%, 14.33%, and 4.16% restoration of cell viability at 0.1, 1, 10, and 50μg/mL, respectively compared to the t-BHP induced group. Further, the treatment groups like one-time Biofield Energy Treated DMEM (BTI) showed 1.51% and two-times Biofield Energy Treated DMEM (BT-II) exhibited 16.28% restoration of cell viability with respect to the t-BHP induced group (Figure 2). Oxidative stress induced by any foreign matters or toxicants causes various complications in the vital organs like liver, heart, kidney, etc. Hence, in the present study, the cardioprotective effect of the Biofield Treated DMEM against oxidative damage induced by tert-butyl hydroperoxide (t-BHP) in H9c2 cells was evaluated in order to correlate in vitro antioxidant activity with cytoprotective effects [34].
Figure 2:
Evaluation of cytoprotective effect of the test substances in H9c2 cells against tert-butyl hydroperoxide (t-BHP) induced cell damage.
TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
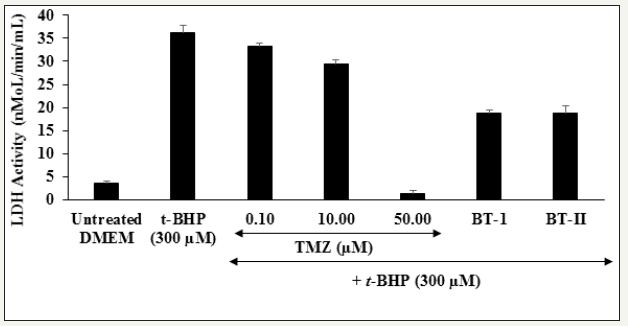
Estimation of Lactate Dehydrogenase (LDH)
For quantitative estimation of the effect of Biofield Treated test items on cell death (necrosis), lactate dehydrogenase (LDH) assay was extensively used by researcher. In this assay, rupture of the cellular membrane is considered as a typical hallmark of necrosis, is quantified in terms of LDH release from the cytosol [35]. The inhibition of lactate dehydrogenase (LDH) enzyme activity after treatment with the Biofield Energy Treated test items and findings are shown in Figure 3. The level of LDH activity was significantly increased by 892.05% in the t-BHP induced group as compared to the untreated DMEM group. Further, the positive control, trimetazidine (TMZ) showed significant reduction of LDH level by 8.04%, 18.64%, and 96.13% at 0.1, 10, and 50μM, respectively compared to the t-BHP induced group. Results of the test substance treatment groups showed that the one-time Biofield Energy Treated DMEM (BT-I) and two-times Biofield Energy Treated DMEM (BTII) groups showed 48.36% and 47.89% reduction of the level of LDH, respectively with respect to the t-BHP induced group (Figure 3). Thus, it is demonstrated that the Biofield Treated test items effectively reduced lactate LDH leakage and reactive oxygen species (ROS) release in t-BHP treated cells, reflecting its ability to reduce t-BHP-induced cell injury.
Figure 3:
The effect of the test substances on lactate dehydrogenase (LDH) against tert-butyl hydroperoxide (t-BHP) induced damage in H9c2 cardiomyocytes.
TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treatment; BT-II: Two-times Biofield Energy Treatment.
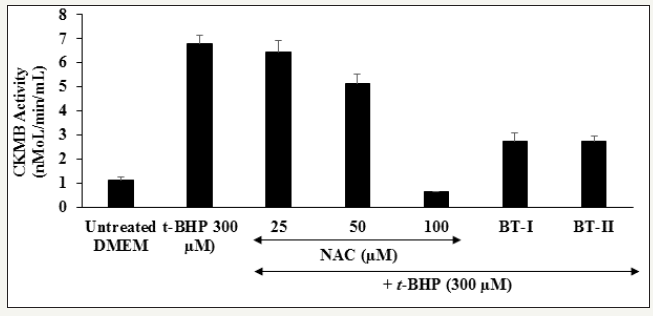
Estimation of Creatine Kinase-Myocardial Band (CK-MB)
The effect of the Biofield Treated test items on cardiac marker, creatine kinase-myocardial band (CK-MB) is shown in Figure 4. The level of CK-MB was significantly increased by 495.61% in the t-BHP induced group as compared to the untreated DMEM group. Moreover, the positive control, N-acetyl cysteine (NAC) showed 4.86%, 24.00%, and 90.57% inhibition of CK-MB enzyme activity at 25, 50, and 100μM, respectively in a concentration-dependent manner compared to the t-BHP induced group. Besides, the experimental groups like one-time Biofield Treated DMEM (BTI) and two-times Biofield Energy Treated DMEM (BT-II) showed 59.5% and 59.5% inhibition of CK-MB enzyme level as compared to the t-BHP induced group (Figure 4). Increased levels of creatine kinase MB (CK-MB) and troponin I (Tn-I) are the vital biochemical markers of myocyte necrosis; that leads to cardiovascular disorders like congestive heart failure (CHF). The clinical manifestation of cardiovascular symptoms is directly proportional to the increased level of CK-MB [36]. In this experiment, the Biofield Energy Treated test items significantly suppressed the level of cardiac marker CKMB, which might be due to The Trivedi Effect® - Consciousness Energy Healing.
Figure 4:
The effect of the test substances on Creatine Kinase-Myocardial Band (CK-MB) activity against tert-butyl hydroperoxide (t-BHP) induced damage in H9c2 cardiomyocytes.
NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM.
Assessment of reactive oxygen species (ROS)
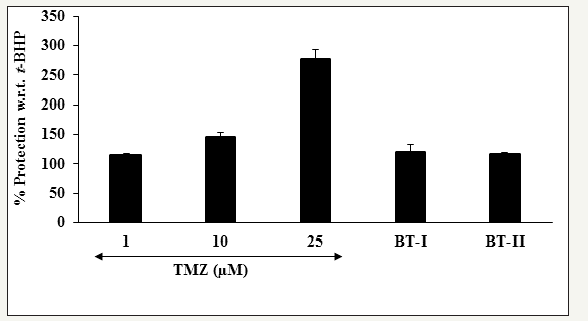
Oxidative stress has been implicated as a major aspect of the pathophysiology of ischemic myocardial injuries, in which ROS generated induce a variety of cellular damages, which is an important risk factor in the pathogenesis of cardiovascular ischemic diseases. Oxidative stress initiation during the reperfusion period is an important risk factors for ischemic injury that leads to cardiovascular disease (CVD) like heart failure, myocardial infarction, and arrhythmia [37]. It gives an indication of an imbalance between formation of ROS and the ability to detoxify the reactive intermediates or to repair the resulting damage [38,39]. The effect of the test items on the ROS induced by t-BHP is shown in Figure 5. The positive control, trimetazidine (TMZ) showed 115.1%, 146.5%, and 277.8% inhibition of ROS at the concentration of 1, 10, and 25μM, respectively. Further, one-time Biofield Energy Treated DMEM (BT-I) showed 120.08% and two-times Biofield Energy Treated DMEM (BT-II) showed 116.20% inhibition of ROS compared to the t-BHP induced group (Figure 5). Results found that the Biofield Treated test items significantly protect cardiomyocytes from oxidative stress.
Figure 5:
The effect of the test substances on the protection of reactive oxygen species (ROS) in H9c2 cells after 24 hours of treatment.
TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
Effect of test item on apoptosis
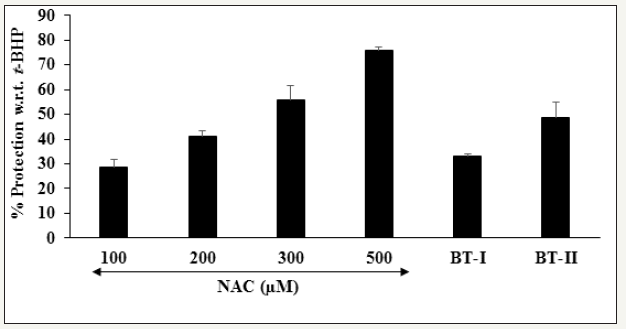
Apoptosis, a programme cell death mechanism that plays an important role in different pathologic conditions related to cardiovascular system [40]. Inhibition of apoptotic pathway is one of the potential therapeutic strategy for the management of cardiovascular disorders [41,42]. The impact of the test items on the suppression of apoptic cells is demonstrated in Figure 6. The positive control, N-acetyl cysteine (NAC) showed a concentrationdependent inhibition of apoptotic cells by 28.5%, 41%, 56%, and 76% at 100, 200, 300, and 500μM, respectively. Moreover, the Biofield Energy Treated test items BT-I (one-time Biofield Energy Treated DMEM) and BT-II (two-times Biofield Energy Treated DMEM) showed 33% and 48.5% inhibition of apoptotic cells, respectively compared to the t-BHP induced group (Figure 6). Overall, results suggested that t-BHP-induced apoptotic cells was significantly inhibited by The Trivedi Effect® - Treated test items.
Figure 6:
Effect of the test substances on the protection of apoptotic cells in H9c2 cells after 24 hours of treatment.
NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
Conclusion
The MTT cell viability assay data showed that the test substances were found as safe and non-toxic with more than 95% viable cells. The two-times Biofield Energy Treated DMEM (BT-II) showed 16.28% cytoprotective activity as compared to the t-BHP induced group. Further, the level of lactate dehydrogenase (LDH) was significantly suppressed by 48.36% in the BT-I group, and 47.89% in the BT-II (two-times Biofield Energy Treated DMEM) group as compared to the t-BHP induced group. The cardio-specific enzyme, creatine kinase-myocardial band (CK-MB) was significantly inhibited by 59.5% in the BT-I and BT-II groups compared to the t-BHP. Moreover, percent protection of reactive oxygen species (ROS) by 120.08% and 116.20% in the BT-I and BT-II groups, respectively, compared to the t-BHP. Apoptotic cells were significantly inhibited by 33% and 48.5% in the BT-I and BT-II, respectively compared to the t-BHP. In conclusion, The Trivedi Effect® - Consciousness Energy Healing Treatment has significantly improved various cardiac parameters and protect cardiomyocytes cells from oxidative stress. Therefore, it can be used as a complementary and alternative treatment for the prevention of various types of cardiac disorders viz. stroke, high blood pressure (hypertension), peripheral artery disease, congestive heart failure (CHF), congenital heart disease, valvular heart disease, venous thrombosis, thromboembolic disease, carditis, and rheumatic heart disease, etc. Further, it could be useful to improve neurotransmission, normal cell cycling and proliferation, cell growth and differentiation, cardiovascular and immune functions. Further, it could be used in organ transplants (i.e., heart, liver and, kidney transplants), aging, hormonal imbalance, and various inflammatory and immune-related disease conditions like Alzheimer’s Disease (AD), Dermatitis, Ulcerative Colitis (UC), Asthma, Diabetes, Hashimoto Thyroiditis, Irritable Bowel Syndrome (IBS), Pernicious Anemia, Hepatitis, Sjogren Syndrome, Multiple Sclerosis, Aplastic Anemia, Graves’ Disease, Atherosclerosis, Dermatomyositis, Myasthenia Gravis, Parkinson’s Disease, Systemic Lupus Erythematosus (SLE), stress, etc.
Acknowledgement
Authors gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, Trivedi testimonials and Trivedi master wellness for their support. In addition, authors are thankful for the support of Dabur Research Foundation for conducting this study.
References
- Rakesh S, Arunporn I (2017) Herbal supplements or herbs in heart disease: Herbiceutical formulation, clinical trials, futuristic developments. J Cardiol Cardiovasc Ther 3(1): 555603.
- (2015) CDC, NCHS. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program.
- (2004) Atlas of Heart Disease and stroke, WHO, USA.
- Peter AK, Bjerke MA, Leinwand LA (2016) Biology of the cardiac myocyte in heart disease. Molecular Biology of the Cell. 27(14): 2149-2160.
- Duthie SJ, Melvin WT, Burke MD (1994) Bromobenzene detoxification in the human liver-derived HepG2 cell line. Xenobiotica 24(3): 265-279.
- Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M (2015) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochimica et biophysica acta 1853(2): 276-284.
- Lutgendorf SK, Houser E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun 24(8): 1231-1240.
- Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, et al. (1996) Massage therapy is associated with enhancement of the immune system’s cytotoxic capacity. Int J Neurosci 84(1-4): 205-217.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4(Supp 1): 58-66.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) in vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-28, 31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. International Journal of Materials Science and Applications 4: 390-395.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7: 83-88.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5(4): 189.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of biochemical marker -Glutathione and DNA fingerprinting of biofield energy treated Oryza sativa. American Journal of BioScience 3(6): 243-248.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Parulkar VR, Trivedi MK, Branton A, Trivedi D, Nayak G et al. (2018) Improved metabolism of vitamin d3 in human osteoblasts cells after biofield energy healing treatment. American Journal of Laboratory Medicine. 3: 11-19.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7: 203-206.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1(2): 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Branton A, Jana S (2017) Effect of The biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH)D3] in rats after a single oral dose of vitamin D3. American Journal of Pharmacology and Phytotherapy 2(1): 11-18.
- Parulkar VR, Trivedi MK, Branton A, Trivedi D, Nayak G et al. (2017) The use of consciousness energy healing based herbomineral formulation for skin anti-aging strategies. Journal of Food and Nutrition Sciences 5(3): 96-106.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. International Journal of Biomedical Engineering and Clinical Science 4: 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). International Journal of Nutrition and Food Sciences 7: 30-38.
- Stutheit ME, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. American Journal of Life Sciences 6: 13- 21.
- Lima CF, Valentao PC, Andrade PB, Seabra RM, Ferreira M et al. (2007) Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem Biol Interact 167(2) :107- 115.
- T MM, Anand T, Khanum F (2018) Attenuation of cytotoxicity induced by tBHP in H9C2 cells by Bacopa monniera and Bacoside A. Pathophysiology 25(2) :143-149.
- Dodo K, Katoh M, Shimizu T, Takahashi M, Sodeoka M (2005) Inhibition of hydrogen peroxide-induced necrotic cell death with 3- amino-2- indolylmaleimide derivatives. Bioorg Med Chem Lett 15(12): 3114- 3118.
- Yilmaz A, Yalta K, Turgut OO, Yilmaz MB, Ozyol A (2006) Clinical importance of elevated CK-MB and troponin I levels in congestive heart failure. Adv Ther 23(6) :1060-1067.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD (2013) Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation 127(1): e6-e245.
- Ramond A, Godin-Ribuot D, Ribuot C, Totoson P, Koritchneva I, et al. (2013) Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam Clin Pharmacol 27(3): 252-261.
- Jeong EM, Liu M, Sturdy M, Ge Gao, Varghese ST, et al. (2012) Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 52(2): 454-463.
- Bennett MR (2002) Apoptosis in the cardiovascular system. Heart 87(5): 480-487.
- Saraste A, Voipio-Pulkki LM, Parvinen M, Pulkki K (1997) Apoptosis in the heart. N Engl J Med 336: 1025-1026.
- Kang PM, Izumo S (2003) Apoptosis in heart: Basic mechanisms and implications in cardiovascular diseases. Trends Mol Med 9(4): 177-182.
© 2019 Snehasis Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






