- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Coronary Artery Disease: Pathogenesis, Progression of Atherosclerosis and Risk Factors
Lamiaa Mageed*
Department of Biochemistry, Egypt
*Corresponding author: Lamiaa Mageed, Department of Biochemistry, National Research Center, Giza, Egypt
Submission: July 20, 2018;Published: November 15, 2018

ISSN 2578-0204Volume2 Issue4
Introduction
Coronary artery disease
Coronary artery diseases (CAD) known as atherosclerotic heart disease, atherosclerotic cardiovascular disease, coronary heart disease (CHD), or ischemic heart disease (IHD) [1]. CAD is the largest contributor of cardiovascular diseases (CVDs) and mortality rate is due in prevalence to atherosclerosis, a chronic inflammatory condition of the arterial wall. Unfortunately, myocardial infarction (MI) is still a first common manifestation of CHD and, in about 50% of patients; angina pectoris is the first symptom of the pathology [2].
Atherosclerosis
Figure 1:Atherosclerotic lesion in a human artery.
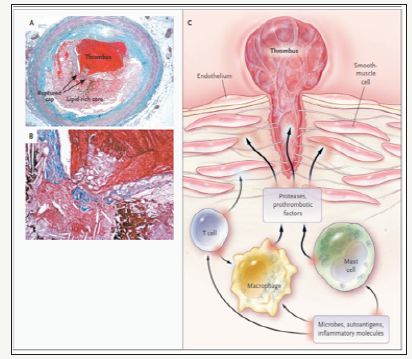
Atherosclerosis is derived from the Greek words ‘athera’ meaning soft gruel-like (porridge-/mush-/paste-like) fatty deposit and ‘sclerosis’ which means hardening. Atherosclerosis is a pathological process that affects large- and medium-sized arteries and causes coronary artery disease (angina pectoris and myocardial infarction), cerebrovascular disease (ischemic stroke and vascular dementia) and peripheral vascular disease (intermittent claudication and gangrene) [3]. Atherosclerosis is a chronic cumulative disease progressing over years. It is characterized by atherosclerotic plaques formed in the wall of the vessels, consisting of necrotic cores, calcified regions, accumulated modified lipids, and inflamed smooth muscle cells (SMCs), endothelial cells, leukocytes, and foam cells (Figure 1). Lesions begin early as fatty streaks and progress into pathologic lesions under the influence of both genetic and lifestyle insults [4,5].
Panel A shows a cross-sectioned coronary artery from a patient who died of a massive myocardial infarction. It contains an occlusive thrombus superimposed on a lipid-rich atherosclerotic plaque. The fibrous cap covering the lipid-rich core has ruptured (area between the arrows), exposing the thrombogenic core to the blood. Trichrome stain was used, rendering luminal thrombus and intraplaque hemorrhage red and collagen blue. Panel B is a high-power micrograph of the area in Panel A indicated by the asterisk and shows that the contents of the atheromatous plaque have seeped through the gap in the cap into the lumen, suggesting that plaque rupture preceded thrombosis (the asterisk indicates cholesterol crystals). (Panels A and B courtesy of Dr. Erling Falk, University of Aarhus, Aarhus, Denmark.)
Panel C illustrates the consequences of the activation of immune cells in a coronary plaque. Microbes, autoantigens, and various inflammatory molecules can activate cells, macrophages, and mast cells, leading to the secretion of inflammatory cytokines (e.g., interferon- and tumor necrosis factor) that reduce the stability of plaque. The activation of macrophages and mast cells also causes the release of metalloproteinases and cysteine proteases, which directly attack collagen and other components of the tissue matrix. These cells may also produce pro-thrombotic and pro-coagulant factors that directly precipitate the formation of thrombus at the site of plaque rupture.
Pathogenesis of atherosclerosis
The pathologist Felix Marchand first introduced the term “atherosclerosis” in 1904, describing the association of fatty degeneration and vessel stiffening [6]. This process affects medium and large-sized arteries and is characterized by patchy intramural thickening of the sub-intima that encroaches on the arterial lumen. Each vascular bed may be affected by this process; the etiology, treatment and clinical impact of atherosclerosis varies from one vascular bed to another [7]. The earliest visible lesion of atherosclerosis is the fatty streak, which is due to an accumulation of lipid-laden foam cells in the intimal layer of the artery (Figure 2). With time; the fatty streak evolves into a fibrous plaque, the hallmark of established atherosclerosis. Ultimately the lesion may evolve to contain large amounts of lipid; if it becomes unstable, denudation of overlying endothelium, or plaque rupture, may result in thrombotic occlusion of the overlying artery [7,8].
Figure 2:Normal heart, normal artery and artery with plaque.
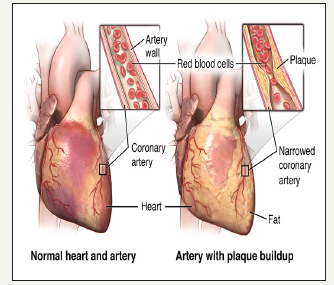
Figure 3:Atherosclerotic plaque formation.
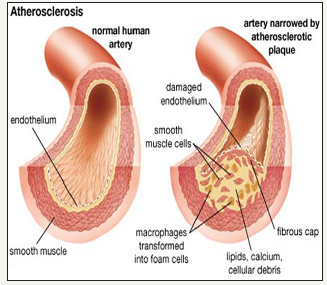
Atherosclerotic lesions (atheromata) are composed of three major components. The first is the cellular component comprised predominately of smooth muscle cells and macrophages. The second component is the connective tissue matrix and extracellular lipid. The third component is intracellular lipid that accumulates within macrophages, thereby converting them into foam cells (Figure 3). Atherosclerotic lesions develop as are sult of inflammatory stimuli, subsequent release of various cytokines, proliferation of smooth muscle cells, synthesis of connective tissue matrix, and accumulation of macrophages and lipid [9,10].
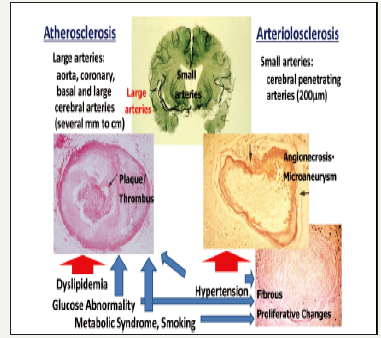
There are 2 major types of vascular pathology leading to stroke, stroke subtypes and IHD. One is atherosclerosis, a large vascular pathology typically observed in the aorta, coronary arteries, carotid arteries and basal cerebral arteries, and characterized by lipid accumulation with proliferative changes leading to plaque formation [11]. The other pathology is arteriolosclerosis, a small vascular pathology typically occurring in small penetrating arterioles in the basal ganglions of the brain, characterized by necrosis or apoptosis of smooth muscle cells within the media, leading to the formation of micro-aneurysms (intra-parenchymal hemorrhage) and fibrous proliferative changes (lacunars stroke) (Figure 4) [11].
Figure 4:Two types of vascular pathology in large and small arteries: Atherosclerosis (Left) and Arteriosclerosis (Right).
Progression of atherosclerosis
Excess generation of (ROS) represents an important pathological process in atherogenesis. Each component of the atherosclerotic blood vessel has been demonstrated to increase production of ROS, primarily superoxide anion (O2-) [12]. Important sources of ROS are vascular smooth muscle cells, endothelial cells, fibroblasts, and infiltrating leukocytes [13]. Production of ROS affects gene transcription, damages DNA, and increases production of inflammatory transcription factors [14]. The two best-characterized effects include oxidation of LDL and scavenging of endothelium-derived NO•. The progression of atherosclerotic disease has been described as moving from an early lesion (phase1) to a more advanced fibro-lipid lesion (phase 2) (Figure 5). The formation of thrombus or hematoma can advance into an acute phase (phase 3 and 4) or even to total occlusion (phase 5). Although there is substantial evidence for this process in the coronary circulation, it is highly likely that it also occurs in peripheral artery disease (PAD). Magnetic resonance imaging (MRI) has allowed better characterization of lesions and has shown the importance of plaque composition to subsequent clinical events [15,16].
Figure 5:Phases of lesion development.

Risk Factors Influencing Coronary Artery Disease
A risk factor can be defined as a characteristic that is associated with increased or decreased likelihood of subsequent development of CVD [17].
Types of risk factors
Non-modifiable risk factors: Risk factors that cannot be prevented, changed or controlled such as: age and gender, because they are not modifiable, they are less determining in terms of risk factor management [17].
A. Age
Ageing is an un-modifiable risk factor for CAD, with males clinically manifesting this condition at 50-65 years of age and females about 10 years later, following menopause. The WHO reports that the principal cause of death of people over 65 years is CAD, and as age increases, a substantial proportion of deaths are among females [18]. In many developed countries, the number and proportion of older people (over 65 years) is increasing, which is largely explained by declines in fertility and mortality. The ageing population of many countries has accelerated the contribution of CAD to total disease burden. It is predicted that the global ageing population will maintain CAD as a predominant cause of death worldwide [18]. Among countries with high but declining CAD mortality, it is suggested that these trends are changing with respect to younger age subgroups [19].
B. Gender
Coronary artery disease is the leading cause of mortality for both adult males and females alike worldwide. Although the initial manifestation of CAD is delayed in females by about ten years compared to males, there is not an abrupt increase in CAD mortality rates for females immediately following menopause but a progressive increase over subsequent years [20,21].
C. Family history
Epidemiological studies indicate that family or parental history of myocardial infarction is a risk factor for coronary heart disease [22]. Nasir et al. [23] examined the association of a reported family history of early-onset (before age 55 years) CHD with the presence and burden of coronary artery calcium (CAC) in electron beam computed tomography in 8549 asymptomatic men and women referred for testing. Coronary artery calcium is a surrogate measure of the presence and burden of coronary atherosclerosis that has consistently been associated with increased risks for CHD in selected cohorts [24]. Coronary artery calcium was significantly more common in subjects with a sibling or parental history of CHD. The elevated odds for CAC conferred by the presence of a family history were not significantly different by strata of individual modifiable risk factors, although the prevalence of CAC in subjects with three or more risk factors was higher in those with than in those without a family history [24]. Zlot et al. [25] have reported that parental history of CHD is associated with increased carotid intima-media thickness, even after adjustment for established risk factors.
Modifiable risk factors: Risk factors that can in principle be prevented, changed, or controlled. Major modifiable risk factors include: sedentariness, smoking, dietary imbalance, impaired glucose tolerance and diabetes mellitus, elevated blood pressure, abnormal blood lipids, and obesity [20].
A. Hypertension (Blood pressure)
Hypertension (HTN), one of the most traditional risk factors, has been consistently correlated with increased probability of developing CAD in various populations [26]. Lewington et al. [26] reported that the Prospective Trialists group each 20/10mm Hg increase in blood pressure (BP) doubles the risk of ischemic heart disease and stroke over the range of 115/75 to 185/115mm Hg in individuals from 40 to 90 years of age. The epidemiological studies are supported by experimental evidence postulating that hypertension predisposes to atherosclerosis through a shared synergistic mechanism involving inflammation and oxidative stress in the arterial wall [27,28].
It is conceivable that the effect of hypertension on CAD onset may be modulated by various environmental and genetic factors. However, it is widely accepted that strategies adapted to lower blood pressure play a protective role by delaying atherosclerotic lesion formation [29]. The association of hypertension with CAD manifestations onset has not been thoroughly investigated in Middle Eastern populations [30,31], showed that there is a significant association between hypertension and acute myocardial infarction (MI) in older patients One study however described hypertension as one of the most frequent risk factors for premature CAD [32].
B. Smoking
Smoking is a major factor in both the development and rate of progression of a cardiovascular disease and coronary artery disease [33]. Nicotine and carbon monoxide contents of cigarette have damaging effects on arteries by causing them to lose their compliance and to set up a stage for plaque development. Cigarette smoking results in high levels of circulating non-esterified fatty acids which can be injurious to the cell by eliciting inflammatory response [34]. The free radicals generated from smoking results in oxidative stress and increases oxidation of LDL which trigger the recruitment of monocytes and T-cells; these lead to formation of macrophages and other processes that promote atherosclerosis. Young et al. [35] explained that the toxins in tobacco smoke lower a person’s HDL while raising levels of LDL cholesterol or “bad” cholesterol. Specifically, smoking has been associated with a twoto six-fold increase in males in the risk for myocardial infarction, a major form of CHD. It has also been associated with a threefold increase in the risk for incident angina. There is a clear dose relationship between CHD and the duration (years) of smoking, the number of cigarettes smoked, the degree of inhalation, and the age of initiation of smoking [36].
C. Type 2 diabetes mellitus (T2DM)
One of the risk factors, diabetes, and its predominant form, type 2 diabetes mellitus (T2DM), has a distinctive association with CHD. The number of people with diabetes mellitus (DM) is increasing due to population growth, aging, urbanization, increasing prevalence of obesity, and physical inactivity, with an estimated number of 200 million patients worldwide [37]. Patients with diabetes have two to four-fold likelihood of developing coronary artery disease with marked morbidity and mortality [38]. CAD in patients with DM is often more advanced at the time of diagnosis compared with patients without DM [39]. In addition, a complex mix of mechanistic processes such as oxidative stress, enhanced atherogenecity of cholesterol particles, abnormal vascular reactivity, augmented haemostatic activation, and renal dysfunction have been proposed as features characteristic of T2DM that may confer excess risk of CHD [40].
D. Obesity (Overweight)
Obesity is a predictor of coronary artery disease both as an independent factor and as a progenitor of the multiple atherogenic processes of the metabolic syndrome [41]. Obesity is associated with an increase in both oxidative stress and the pro-inflammatory effects of certain cytokines. Interleukin-6 is produced in adipocytes and increasing adipocyte mass causes an elevated production of IL-6. These higher levels of IL-6 subsequently stimulate production of C-reactive protein in the liver and both play a role in endothelial dysfunction by decreasing nitric oxide (NO•), leading to vasoconstriction and increasing vascular resistance [42]. This association remained significant even after adjusting for other risk factors (non-HDL, HDL, smoking and hypertension). These data lead to the ominous realization that obesity in adolescents and young adults accelerates the progression of atherosclerosis decades before the onset of clinical symptoms [43].
E. Dyslipidemia (Hypercholesterimia)
A high level of LDL-C in the blood is the principal cause of injury to the artery and vascular SMCs [44]. With high levels of LDL in vascular endothelium, leukocytes start to ‘cling’ to the endothelium and cause further accumulation of lipids which result in foam cells formation. Abnormalities in the regulatory mechanisms of LDLreceptors and fatty diet can result in hypercholesterimia which can eventually lead to atherosclerosis [35]. High density lipoproteincholesterol (HDL-C) or “good” cholesterol inhibits oxidative modification of LDL and blocks the pro-inflammatory effects of oxidized LDL (ox-LDL) [44]. HDL provides protection against atherosclerosis by promoting the activity of antioxidant enzymes like platelet activation factor, acetyl hydrolase and paraoxonase [45].
F. Sedentariness
Physical activity is a key determinant of energy expenditure and thus fundamental to energy balance and weight control. Physical activity improves endothelial function, which enhances vasodilatation and vasomotor function in the blood vessels [46]. In addition, physical activity contributes to glycaemic control, improved blood pressure, lipid profile and insulin sensitivity [47]. The protective value of physical activity is independent of measures of total cardiovascular risk such as the score estimated using the Framingham risk equation [48].
Other factors are also of importance: psychosocial, such as perceived stress at work, symptoms of depression, low socioeconomic status, as well as indicators of chronic inflammation and haemostatic factors [17].
A. Psychosocial factors
Psychosocial factors contribute independently to the risk of CHD, even after statistical control for the effects of standard risk factors. These factors may act as barriers to treatment adherence and efforts to improve lifestyle, as well as promoting health and well-being in patients and populations [49]. Low socioeconomic status, lack of social support, social isolation, stress at work and in family life, negative emotions including depression and hostility have been shown to influence both the risk of contracting CHD and the worsening of clinical course and prognosis in patients with CHD. Several behavioral and psycho-physiological mediators and moderators of these effects have been identified [48].
B. Socio-economic status
Social determinants such as the distribution of income or the level of education indirectly influence cardiovascular health as well as health in general. These determinants shape a set of socioeconomic positions within hierarchies of power, prestige and access to resources. Several structural mechanisms are responsible for creating the differential social positions of individuals, including governance, education systems, labor market structures and the presence or absence of redistributive welfare policies. Social stratification shapes individual health status as well as CVD outcomes by impacting behavioral and metabolic cardiovascular risk factors, psychosocial status, living conditions and the health system [50].
The final report of the Commission [20] made three overarching recommendations: (i) to improve daily living conditions; (ii) to tackle the unequal distribution of power, money and resources; and (iii) to monitor health inequities. WHO Member States discussed the report and passed a resolution urging action on social determinants at the 2009 World Health Assembly (WHA) [20]. The resolution called for a “Health in All Policies” approach and a renewed commitment to intersect oral action to reduce health inequities as well as the implementation of a social determinants approach across public health programs. Poverty, low rates of literacy, environmental degradation, poor housing and unplanned urbanization have a negative impact on health [51].
References
- Eagle K (2008) Coronary artery disease in India; challenges and opportunities. Lancet 371(9622): 1394-1395.
- Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS (2014) The epidemic of the (20th) century: Coronary heart disease. Am J Med 127(9): 807-812.
- Shanmugma N, Román-Rego A, Ong P, Kaski JC (2010) Atherosclerotic plaque regression fact or fiction? Cardiovasc Drugs Ther 24(7): 311-317.
- Manolis SA, Manolis TA, Melita H (2012) Atherosclerosis: An atherothrombo- inflammatory disease. Hospital Chronicles 7(4): 195-209.
- Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16): 1685-1695.
- Mehta NJ, Khan IA (2002) Cardiologys 10 greatest discoveries of the 20th century. Tex Heart Inst J 29(3):164-171.
- Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, et al. (2004) Atherosclerotic vascular disease conference: writing group III: Pathophysiology. Circulation 109(21): 2617-2625.
- http://www.hopkinsmedicine.org/healthlibrary/conditions/ cardiovascular
- Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347): 317-325.
- http://www.human physiology.wikispaces.com
- Iso H (2011) Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb 18(2): 83-88.
- Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49(5): 835-842.
- Fukai T, Ushio Fukai M (2011) Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6): 583-606.
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators of Inflammation Article ID 453892, p. 11.
- Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, et al. (2010) Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the heinz nixdorf recall study. J Am Coll Cardiol 56(17): 1397-1406.
- Corti R, Fuster VJ (2004) Thromb Thrombolysis 17: 35-44.
- Guy G, Backer D (2008) Risk factors and prevention of cardiovascular. Dialogues in Cardiovascular Medicine 13(2): 83-99.
- Christus T, Shukkur AM, Rashdan I, Koshy T, Alanbaei M, et al. (2011) Coronary artery disease in patients aged 35 or less - a different beast? Heart Views 12(1): 7-11.
- David S, Jeremy A (2013) The decline and rise of coronary heart disease: understanding public health catastrophism. Am J Public Health 103 (7): 1207-1218.
- World Health Organization (2009) Global health risks: Mortality and burden of disease attributable to selected major risks. WHO, Geneva.
- Wake R, Takeuchi M, Yoshikawa J, Yoshiyama M (2007) Effects of gender on prognosis of patients with known or suspected coronary artery disease undergoing contrast-enhanced dobutamine stress echocardiography. Circ J 71(7): 1060-1066.
- Walter FM, Emery J (2006) Perceptions of family history across common diseases: A qualitative study in primary care. Fam Pract 23(4): 472-480
- Nasir K, Michos ED, Rumberger JA, Braunstein JB, Post WS, et al. (2004) Coronary artery calcification and family history of premature coronary heart disease: sibling history is more strongly associated than parental history. Circulation 110(15): 2150-2156.
- Hoseini K, Sadeghian S, Mahmoudian M, Hamidian R, Abbasi A (2008) Family history of cardiovascular disease as a risk factor for coronary artery disease in adult offspring. Monaldi Arch Chest Dis 70(2): 84-87.
- Zlot AI, Valdez R, Han Y, Silvey K, Leman RF (2010) Influence of family history of cardiovascular disease on clinicians preventive recommendations and subsequent adherence of patients without cardiovascular disease. Public Health Genomics 13(7-8): 457-466.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360(9349): 1903-1913.
- Li JJ, Chen JL (2005) Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses 64(5): 925-929.
- Okeefe JH, Carter MD, Lavie CJ (2009) Primary and secondary prevention of cardiovascular diseases: A practical evidence-based approach. Mayo Clin Proc 84(8): 741-757.
- Tropeano AI, Saleh N, Hawajri N, Macquin Mavier I, Maison P (2011) Do all antihypertensive drugs improve carotid intima-media thickness? A network meta-analysis of randomized controlled trials. Fundam Clin Pharmacol 25(3): 395-404.
- Sengul C, Ozveren O, Cevik C, Izgi C, Karavelioğlu Y, et al. (2011) Comparison of psychosocial risk factors between patients who experience acute myocardial infarction before and after 40 years of age. Turk Kardiyol Dern Ars 39(5): 396-402.
- Zuhdi AS, Mariapun J, Mohd Hairi NN, Wan Ahmad WA, Abidin IZ, et al. (2013) Young coronary artery disease in patients undergoing percutaneous coronary intervention. Ann Saudi Med 33(6): 572-578.
- Sadeghi R, Adnani N, Erfanifar A, Gachkar L, Maghsoomi Z (2013) Premature coronary heart disease and traditional risk factors-can we do better? Int Cardiovasc Res J 7(2): 46-50.
- Piano MR, Benowitz NL, Fitzgerald GA, Corbridge S, Heath J, et al. (2010) Impact of smokeless tobacco product on cardiovascular disease: Implications for policy, prevention, and treatment: a policy statement from the American heart association. Circulation 122(15): 1520-1544.
- Young JL, Libby P (2007) Atherosclerosis. In: Lilly LS (Edn.), Pathophysiology of heart diseases: A collaborative project of medical student and faculty (6th edn), Lippinocolt Wiiliams & Wilkins, Baltimore, Philadelphia, Pennsylvania, USA, pp. 1-467.
- Young A, Koduri G, Batley M, Kulinskaya E, Gough A, et al. (2007) Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 46(2): 350-359.
- Woodward M, Lam T, Barzi F, Patel A, GU D, et al. (2005) Smoking, quitting, and the risk of cardiovascular disease among women and men in the Asia-Pacific region. Int J Epidemiol 34(5): 1036-1045.
- International Diabetes Federation (IDF) (2009) Diabetes atlas (4th edn).
- Rana JS, Dunning A, Achenbach S, Al Mallah M, Budoff MJ, et al. (2012) Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: Results from 10,110 individuals from the confirm (coronary CT angiography evaluation for clinical outcomes): An International multicenter registry. Diabetes Care 35(8): 1787-1794.
- Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, et al. (2007) Diabetes and mortality following acute coronary syndromes. JAMA 298(7): 765-775.
- Barnett AH (2008) The importance of treating cardiometabolic risk factors in patients with type 2 diabetes. Diab Vasc Dis Res 5(1): 9-14.
- Joseluis I (2009) Obesity and cardiovascular disease. The Journal of Lancaster General Hospital 4(4): 130-133.
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, et al. (2006) Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the Council on nutrition, physical activity, and metabolism. Circulation 113(6): 898-918.
- Gomes F, Daniela F, Heraldo P, José C, Halpern A, et al. (2010) Obesity and coronary artery disease: Role of vascular inflammation. Arq Bras Cardiol 94(2): 255-261.
- Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83 (2): 456S-460S.
- Sotiriou SN, Orlova VV, Al Fakhri N, Ihanus E, Economopoulou M, et al. (2006) Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J 20(3): 559-561.
- Cornelissen VA, Fagard RH (2005) Effect of resistance training on resting blood pressure: A meta-analysis of randomized controlled trials. J Hypertens 23(2): 251-259.
- Kelley GA, Kelley KS, Vu Tran Z (2005) Aerobic exercise, lipids and lipoproteins in overweight and obese adults: A meta-analysis of randomized controlled trials. Int J Obes (Lond) 29(8): 881-893.
- Hu G, Tuomilehto J, Borodulin K, Jousilahti P (2007) The joint associations of occupational, commuting and leisure-time physical activity and the Framingham risk score on the 10-year risk of coronary heart disease. Eur Heart J 28(4): 492-498.
- Grundy S (2005) Prevention of atherosclerotic cardiovascular disease: why are the benefits of lifestyle therapies neglected? Dialogues Cardiovasc Med 10: 73-88.
- World Health Organization (2010) Equity, social determinants and public health programmes. WHO, Geneva, USA, p. 291.
- Tamayo T, Christian H, Rathmann W (2010) Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: A systematic review. BMC Public Health 10: 525-528.
© 2018 Lamiaa Mageed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






