- Submissions

Full Text
Open Access Research in Anatomy
Correlation of Bone Density in Aquatic and Semiaquatic Animals to Ecological and Dietary Specializations
Sulman J Rahmat, Madelyn G Crowell* and Irina A Koretsky
Department of Anatomy, USA
*Corresponding author: Madelyn Crowell, Laboratory of Evolutionary Biology, Department of Anatomy, Washington DC, USA
Submission: December 13, 2019Published: January 27, 2020

ISSN: 2577-1922
Volume2 Issue3
Abstract
One of the most obvious adaptations of animals reintroduced to an aquatic environment is the difference in bone density. Numerous marine mammals and marine reptiles exhibit changes in bone density that correlate to their habitat (ecological niche) and dietary specializations, not phylogenetic relationships. Increased bone density (either pachyostosis, osteosclerosis, or pachyosteosclerosis) was observed early in the transition of terrestrial taxa to the aquatic environment. Animals such as early cetaceans and sirenians clearly exhibit these adaptive features and even retain many terrestrial characters such as hind limbs and behaviors such as paddle swimming). The increase in bone density is a more energetically efficient hydrostatic mechanism for buoyancy for marine mammals with large lung volumes. As the taxa became more specialized for the aquatic environment morphologically (evolving fins, flippers, and flukes) and behaviorally (evolving an oscillating swimming mechanism), variation in bone density correlates with their ecological niche. Modern sirenians retain increased bone density, allowing these large-sized mammals to remain submerged in shallow waters to feed on sessile littoral foods (sea grasses). However, the bone density in modern cetaceans became more osteoporotic, allowing them to swim faster and hunt faster moving prey. Pinnipeds live in a wide range of habitats (from cold to warm waters) and demonstrate varying feeding mechanisms, ranging from filter feeding on krill, bottom feeding on mollusks, and/ or catching fast moving prey. Bone density is one vital character that can be used to predict the specific ecological niche and feeding preference for pinnipeds. Some early hominids have been shown to have an increase in bone density. These heavier, thicker bones would make it easier for early Homo to hunt in waters for littoral food sources and would compensate for the lack of stability from bipedalism.
Keywords: Pachyosteosclerosis; Osteosclerosis; Pachyostosis; Bone density
Introduction
Pachyosteosclerosis combines the osteological conditions of osteosclerosis and pachyostosis. Osteosclerosis is defined as the increase of compact bone in the medullary region in place of spongy bone and/or the filling in of the medullary cavity resulting in an increased density [1], while pachyostosis refers to swollen or thickened bone (Figure 1). Osteosclerosis (Figure 1A) can be associated with the endosteum, whereas pachyostosis (Figure 1D) shows an increase in the compact bone of the periosteum. Pachyostosis results in outward expansion of cortical bone that increases bone density due to enlarged crosssectional thickness [1]. Osteosclerosis and pachyostosis can either occur independently or simultaneously, as an aquatic adaptation for different taxa [1-3].
Figure 1: 3D micro CT scans of bone microanatomy. (A) Normal bone compared to (B) Osteosclerotic and (C) Osteoporotic bone in longitudinal sections (Modified from Dion & Ste-Marie, 2012). (D) Cross-sectional view of pachyostotic bone (Modified from Klein et al., 2016).

Bone is a composite material that consists of minerals such as hydroxyapatite that provides compression strength and collagen that provides tensile strength. Therefore, an increase in the mineralization such as that in osteosclerosis, results in brittle bone prone to fracture [3-5] tested the fracture toughness of manatee ribs, which are highly mineralized and pachyosteoslcerotic [6,7], and compared them to bovine and human bones, both of which are much less mineralized. Manatees have less fracture toughness when compared to less mineralized terrestrial animals [4,5]. Therefore, because of the increased likelihood of fracture dissemination, osteosclerosis with accompanying hyper mineralization is probably not best suited for terrestrial locomotion [8].
Figure 2: Trabecular Density. Photo of proximal portion of a human femur cut longitudinally to show trabecular bone (Modified from Safadi et al., 2009).

Figure 3: Increased Compact Bone. Caudal view of the distal portion of a right partial humerus from the extinct cystophorine seal Pachyphoca chapskii (Modified from Koretsky & Rahmat 2013). Note that the spongy bone of the medullary region has been replaced by compact bone

Historically, pachyostosis was the term used for all hypertrophic conditions, with osteosclerosis recognized as a separate phenomenon. The term osteosclerosis is a blanket term that encompasses events with different causes [9]. For example, increased trabecular density could mean an increase in the total number of trabeculae within a sample or an increase in the density of each individual trabecula within bone. Regardless, both forms of increased trabecular density could result in the loss of a true medullary cavity. The network of trabeculae in the medullary region is commonly referred to as spongy bone (Figure 2). An increase in spongy bone resulting in the loss of the medullary cavity has previously been called osteoporosis due to the assumption that there was a decrease in bone density [9]. Osteosclerosis also covers the increase in compact bone towards the endosteal area that encroaches on the medullary cavity. In extreme cases, the medullary cavity is completely filled with compact bone (Figure 3).
The last large-scale review detailing increased bone density in aquatic animals by Houssaye [3] used the term pachyostosis in quotations due to inconsistency in term definition. An updated review is necessary to include more recently discovered extinct taxa to fill in the gaps of the fossil record as well as extant taxa recently subjected to studies in bone microanatomy. This review should serve as an initial study for researchers interested in the correlation between bone density and ecological specialization in aquatic and semiaquatic animals.
Bone density in fully aquatic animals
Based on body weight, aquatic animals have higher overall volume than their terrestrial counterparts due to lung volume and large amounts of subcutaneous body fat (blubber). This higher overall volume results in a positive buoyancy and allows for aquatic animals to float and ascend without utilizing extra energy. However, this increased overall volume also results in a decreased density, making diving less energy efficient [10]. Increased bone density is one mechanism that some aquatic and semi-aquatic mammals use to counteract this increase in volume and buoyancy. The amassed bone density results in an increase in body mass and inertia, which then leads to a decrease in acceleration, maneuverability, as well as buoyancy [11,12]. Some deep diving, large lunged cetaceans, such as dolphins, have lighter osteoporotic bones, resulting in a positive buoyancy. During those deep dives, the lungs are compressed with oxygen stored in other places such as the blood [3,13-16]. This reduced lung volume at deep depths causes an elevation in the overall density of the animal, as well as a negative buoyancy making increased bone density unnecessary [2]. However, in the shallow diving sirenians (sea cows and dugongs), the lungs remain inflated causing an increase in overall body volume and a reduction in overall body density. While their large lung volume allows them to graze for longer periods of time on a single breath, the large lung volume of these animals also result in an increase in buoyancy. The presence of thick, heavy bones counteracts the increased buoyancy associated with the large lung compacity and enables these animals to remain close to their food source on the sea floor without expending extra energy [1,3,17-19].
The specific location of higher density bones is vital for the stability of the animal’s body trim. Pachyosteosclerosis is more commonly found in the ribs and long bones, ensuring that animals such as sirenians remain in the proper dorsal-ventral position even when at rest and are not forced to expend energy resisting buoyancy. Some modern cetaceans have specialized dorsal fins that allow them to remain in an upright position, negating the need for heavy ventral bones. Animals that have neither dorsal fins nor heavy ventral bones are forced to expend extra energy to remain upright by consistently re-positioning the fins or tail [17,20].
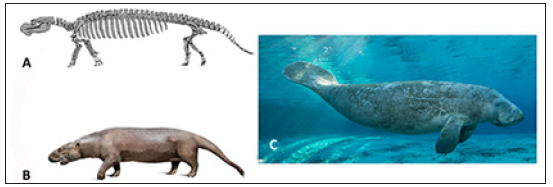
Sirenian bone microanatomy is well documented in both fossil and modern taxa [3,9,21-24], with increased bone density retained in all species. Most early sirenians were pachyosteosclerotic, except for Pezosiren portelli (early Middle Eocene, ~ 50mya) which had only pachyostotic bones and Protosiren (Middle Eocene, 47.8- 37.8mya) which only had osteosclerotic bones [1]. Pez. portelli (found in Jamaica) and Protosiren (found in Egypt and Pakistan) not only represent different sirenian clades of an earlier time period, but also basal and intermediate body types, respectively. Pez. portelli (Figure 4A & 4B) had features, such as hind limbs and the sacroiliac joint that were similar to its terrestrial Tethytherian ancestors (such as elephants) and only bone microanatomy shows evidence of an aquatic lifestyle. Thus, pachyostosis precedes osteosclerosis in fossil sirenians. Modern sirenians have elongated lungs with increased volume, when compared to terrestrial mammals, that aid in the maintenance of body trim [7]. They also retain pachyosteosclerosis, enabling them to feed on the low energy sea grasses and rhizomes on the sea floor at shallow depths and aids in body trim as well without increasing energy expenditure [1,7].
Figure 4: Sirenians. Artist rendering of the (A) skeleton (Modified from Domning, 2001) and (B) possible body phenotype of the extinct Pezosiren portelli. (C) Modern Sea Cow (Trichechus) are more specialized for the aquatic environment with the modification of limbs to flippers and flukes (Photo credit Carlton Ward Jr. for Visit Florida).
Pachyosteosclerosis in sirenian clades has been very well studied [1,7,21-25]. The mechanism in which pachyosteosclerosis has been achieved in modern sirenians has been maintained since the Eocene. However, there is evidence that during the Eocene, not all sirenians achieved pachyosteosclerosis in the same way or at the same time. Osteosclerosis in modern sirenians (as well as many other marine mammals) is achieved by the deposition of lamellar bone in the space between trabeculae and a loss of osteoclastic activity. All modern and most extinct sirenians also continue to have calcified cartilage present in the diaphysis of bone well into adulthood due to incomplete endochondral ossification [25].
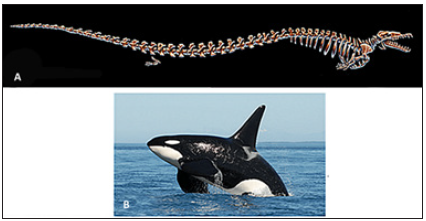
The bone microanatomy of fossil cetaceans was similar to sirenians, but most modern cetaceans do not retain increased bone density [3,15,16,18]. A basal ancestor of cetaceans, Pakicetus (Early Eocene, 56-47.8 mya), had increased bone density and retained some terrestrial features, such as legs and a strong sacro-illiac joint [16,26]. The dense bone found in the limbs likely correlated with anti-buoyancy in shallow waters where these animals may have fed [16,22,27]. While sirenians underwent pachyostosis early on, cetaceans originally began with osteosclerosis. By the time the fully aquatic cetacean Basilosaurus (Figure 5A) appeared in the Middle Eocene (47.8-37.8 mya), pachyosteosclerosis was found in their ribs [16]. Basilosaurus cetoides is known to have likely been a slow swimming species not only due to the increased bone density but also due to the inconspicuous vertebral muscle attachments associated with aquatic locomotion, as well as the lack of streamlining in the limbs [28]. Also, during this evolutionary time, cetaceans had become more specialized for aquatic life (developing fins, flippers and flukes) and many expanded their habitat to deeper oceans with faster moving prey. These animals no longer employ the slower paddle swimming mechanism of their terrestrial and semi-aquatic ancestors; this method is exchanged for a faster, more energy efficient oscillatory or undulatory swimming technique that is associated with animals that are more specialized for the marine environment [16,29]. Heavier, thicker bones became disadvantageous for these cetaceans, leading to an osteoporotic state enabling faster swimming and deeper diving to catch quicker open sea prey. Many cetaceans also began to employ an oscillating hydrodynamic mechanism of swimming, diving, and surfacing that is more energy efficient. During deep dives, cetaceans evolved the ability to collapse the lungs as a mechanism against buoyancy. These adaptations are still present in most modern taxa [1,15,16,18].
Figure 5: Cetaceans. (A) Ancestral pachyosteosclerotic Basilosaurus and (B) the modern Killer Whale (Orcinus orca; Photo credit: Center for Whale Research).
Cetacean bone microanatomy has been studied quite extensively as well [15,16,18,26]. The mechanism in which cetaceans achieve the osteoporotic state seen in modern taxa is through diffuse and localized overactive resorption. Generally, the cortical bone is deposited normally but soon thereafter resorbed until it appears cancellous. Resorption continues throughout the life of the animal and is the predominate process [30,31].
Clementz et al. [22] further tested the validity of the assumption that bone microanatomy is associated with aquatic adaptation by measuring oxygen and carbon isotopes in both early sirenians and cetaceans to determine habitat and diets of these animals that retained terrestrial features. Although the specific habitat for sirenians was not determined due to the low numbers of animals examined, the Clementz et al. [22] study confirmed that sirenians ate marine sea grasses early in their evolutionary history and invaded freshwater environments later. It was determined that pakicetids were likely aquatic animals that fed on terrestrial foods, perhaps similar to extant crocodiles, or freshwater foods. This result is contrary to the morphological analysis that suggested that Pakicetus were likely running and jumping terrestrial animals based on the post-cranial skeletal anatomy [26]. The authors also determined that Basilosaurus was likely fully marine animals that likely fed close to the shore. These tests confirm that bone microanatomy can be a reliable indicator of aquatic adaptations even when morphology is ambiguous [22].
Figure 6: Variation in Bone Density in Sympatric Modern Seal Species. (A) The modern Harp Seal (Pagophilus groenlandicus) is a deep diving, fast moving species with corresponding bone microanatomy (Photo credit: Henry Ausloos) (B) of the humerus (sagittal section) that has increased spongy bone. (C) The modern Ringed Seal (Pusa hispida) is a slow moving, shallow swimming species (Photo found at www.ecomare.nl). (D) The humerus (sagittal section) of this seal has compact bone replacing the spongy bone causing an increase in density (Bone sections modified from Rahmat and Koretsky, 2017).
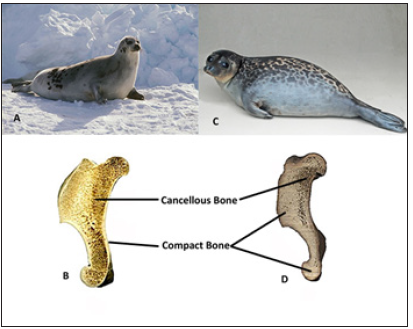
Taxonomically close species of fish living in a similar environment have been shown to have differences in bone density that likely corresponds to diving depth and hunting speed. In the case of the extinct teleost Aphanius crassicaudus, two distinct phenotypes are believed to have lived sympatrically: one pachyostotic and another non-pachyostotic [32]. The nonpachyostotic teleost would likely have been able to swim faster, feeding on a diet different than the heavier, slower pachyostotic fish that likely lived in shallower waters, maintained a more constant depth and caught prey at that level. This is also seen in the modern harp seal (Pagophilus groenlandicus, Figure 6A & 6B) and ringed seal (Pusa hispida, Figure 6C & 6D), sympatric species that differ in bone density. The harp seal has lighter bones and is known to swim faster and dive at deeper depths than the ringed seal. The fast, deep diving harp seal can feed on prey at the same depth as the ringed seal as well as faster prey at deeper depths, expanding their potential hunting area. This is different than the heavierboned ringed seal that can only hunt slow moving prey and have limited dietary range [14,19,33]. The harp seal would be able to dive deeper but would not be able to remain at a constant depth without expending extra energy due to buoyancy pushing against their lighter bones; opposite to ringed seal that does not usually dive deep but can hydrostatically maintain depth due to their denser bones.
Bone density of some semi-aquatic vertebrates
Bone density in semi-aquatic animals is much more complex due to the lack of a true definition for the terms “semi-aquatic” or “amphibious,” as well as the need of those animals to efficiently move on both land and water [34]. Gemain & Laurin [35] categorize species such as the Southern elephant seal (Mirounga leonina) and the Southern sea lion (Otaria byronia) as aquatic species and the American mink (Mustela vison) as a terrestrial species [35]. According to Cooper et al. [36], semi-aquatic could refer to several behaviors such as water feeding, escaping to water, habitual wading, and typically submerged. The proclivity for these “semi-aquatic” behaviors also affected the results of inference models using bone microanatomy. Thus, only species that spent increased time in water than on land can be accurately separated from terrestrial species because amphibious taxa are more adapted to the aquatic environment [36]. However, there is a trend towards higher bone density in most semi-aquatic species compared to their terrestrial counterparts, with those living in the shallows typically having dense bones, but lighter bones in active deep divers similar to those seen in most modern cetaceans [35].
Reptiles: Placodonts were extinct, slow moving, bottom-feeding reptiles who had few osteological adaptations for the aquatic habitat they occupied [3,16]. Examination of placodont dentition revealed signs of wear due to eating hard shelled mollusks [3]. Placodonts displayed an increase in bone density in their skulls and some long bones, but without equal distribution. Houssaye et al. [16] described increased bone density in the humeri of placodonts, more so than in the femora, possibly due to specialization of the humeri during feeding.
A study by Houssaye [37] on squamates (reptiles) from the Late Cretaceous period (100.5-66mya) examined the degree and type of bone microanatomy and the sediment in which the squamates were found, detailing the likely environment in which these animals lived. Squamates who had increased bone density in the axial skeleton likely inhabited shallow waters, while those without an increase in density lived in more diverse marine environments. Similar to recent and extinct marine mammals, the increased bone density in squamates would counteract buoyancy and increase stability when submerged. This was found to likely be the case for the aquatic squamate Pachyvaranus crassispondylus that was found in the shallow marine deposits and had increased bone density in the vertebrae [8] as well as Pachyophis woodwardi, an extinct snake with increased bone density and a laterally flattened body in the caudal region [38]. Unlike mammals, most extant snakes living in different environments show less variation in the bone density of the axial skeleton and maintain a generalist bone microanatomy to fit diverse environments [8,37,39]. So, while there is no difference in bone density between modern climbing and terrestrial squamates, there is a trend towards increased density between terrestrial (lowest), aquatic (intermediate) and burrowing (highest) taxa [1].
Figure 7: Crocodilian body plan. (A) Skeleton of the extinct aquatic reptile Champsaurus natator (Photo by D. Gordon E. Robertson at the Canadian Museum of Nature, Ottawa, Ontario). Images of (B) the Dwarf (Osteolaemus tetraspis; Photo from San Diego Zoo) and (C) Nile (Crocodylus niloticus; Photo from South African National Biodiversity Institute) crocodiles. All had similar body plans that are associated with comparable hunting techniques. The Nile crocodile has a more aquatic lifestyle than its close relative the Dwarf crocodile and therefore has an increase in bone density
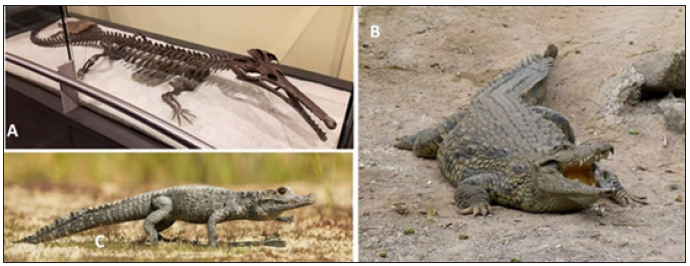
Champsosaurids (Figure 7A) and Simoedosaurids are both extinct aquatic reptiles whose body plans resemble modern day crocodilians showing that the crocodilian body plan was more advantageous in their specific ecological niches. To maintain antibuoyancy and dorsal-ventral position stability, these animals had pachyosteoclerotic ribs, vertebrae, and long bones [3]. Modern crocodilian species live in shallow waters and can hunt from the riverbed. Unlike other animals that remain in shallow environments, crocodiles are not bottom feeders. Instead, crocodiles have increased bone density corresponding to the degree to which they live in the water and not to phylogenetic relationships. The Nile crocodile (Crocodylus niloticus; Figure 7B) has a higher bone density than its closest relative, the dwarf crocodile (Osteolaemus tetraspis; Figure 7C), which is the least aquatic of the two species [24]. However, the increased density in the Nile crocodile is moderate when compared to other shallow water animals because this species also has other mechanisms that assist in increasing overall body density, such as their bony scuta [35].
Birds: Bone density in birds corresponds to four behaviors: flight, flight and diving, diving without flight and terrestrial without flight [24]. Birds in the flight group, such as the Common buzzard (Buteo buteo) and the Gray heron (Ardea cinerea), tend to have the lightest bones because it is energetically inefficient to fly with heavy bone. Terrestrial flightless birds such as the Southern cassowary (Casuarius casuarius) and the Common ostrich (Struthio camelus) have higher bone density than flight birds. The densest bones [24] are those flightless birds that also dive, such as the shallow Humboldt penguin (Spheniscus humboldti), and those that dive with flight such as the Northern gannet (Morus bassanus). Therefore, increased bone density is associated with diving in birds [24].
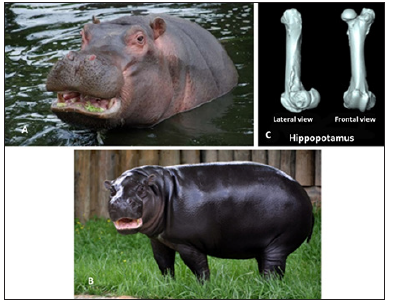
Mammals: Hippopotamuses are semiaquatic animals that spend a considerable portion of time in the water. Their large size (large amounts of subcutaneous fat) and large lungs would cause buoyancy instability while in water. However, very similar to what is seen in sirenians, hippopotamuses are likely able to control buoyancy and dorsal-ventral orientation with the presence of pachyostotic bones. These heavy bones are disadvantageous to terrestrial animals, making them slow and more susceptible to predation [24]. The common hippopotamus is also considered a graviportal animals similar to elephants and rhinoceros which also have increased bone density more similar to osteosclerosis [18]. Houssaye et al. [18] says that it is impossible to know whether this increase in density is due to graviportal adaptations, aquatic lifestyle, or a combination of both [18]. Whether this increase in density is a graviportal or aquatic adaptation, the common hippopotamus (Figure 8A) is most closely related to the pygmy hippopotamus (Figure 8B) yet the pygmy hippopotamus has lighter bones either because they have a more terrestrial lifestyle or because pygmy hippopotamus are not considered graviportal [18,24]. This difference in bone density (Figure 8C), like the crocodilians mentioned earlier, is not likely related to phylogenetic relationships.
Figure 8: Hippopotamuses. (A) The Nile or common hippopotamus (Hippopotamus amphibious; Image from Zoological Society of London) has a more aquatic lifestyle and increased bone density than (B) the pygmy hippopotamus (Choeropsis liberiensis; Photo courtesy of metro Richmond zoo). (C) CT Scan of the Nile hippopotamus femur showing thickened bone (Image found at http://mammals locomotion.com/index.html).
Otters share many similarities with terrestrial mammals [20] but spend majority of their lives in the water. Puijila darwini, thought to have been either a fossil mustelid or an ancestor of pinnipeds, likely had semiaquatic lifestyle and increased density in their long bones [40]. Modern river otters (Lontra canadensis; Figure 9A) actively hunt for fish and regularly pull themselves and their prey out of the water in order to feed. Extremely heavy bones are not advantageous for animals that spend a considerable amount of time on land, therefore, river otters have an intermediate density between terrestrial mustelids and the more aquatic Sea otters. In contrast, Sea otters (Enhydra lutris; Figure 9B) spend a majority of their time in the shallow waters eating urchins, abalone, crabs, clams and mussels but don’t have to leave the water for feeding. Instead, they eat while floating on their backs [41]. Sea otters are more adapted to the water due to the increased time spent in the aquatic environment compared to river otters. One such adaptation is a tendency towards osteosclerosis in their ribs and limbs [1,2,42]. The increased density seen in sea otters allow for their diving behaviors and harvesting of benthic organisms to be more energy efficient. However, this microanatomy is cumbersome in the terrestrial environment and makes water parturition for sea otters an advantage when they are trying to avoid predation and human hunting for fur.
Figure 9: Otters. The (A) river otter (Lontra canadensis; Photo credit Marcus Sharpe) and (B) sea otter (Enhydra lutris; Photo credit: Neil Fisher) differ in lifestyle as well as bone density. The sea otter has a more aquatic lifestyle and increased bone density when compared to the river otter. There is no visual difference in bone morphology between the humerus (Images from Idaho Virtual Museum) of a river otter (C) compared to the osteosclerotic humerus of a sea otter (D).
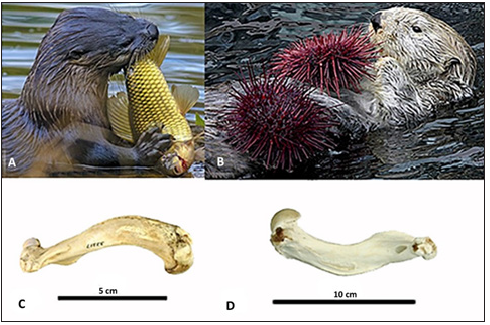
Figure 10: Odobenidae. (A) Walruses (Odobenus rosmarus; Image from Marine Mammal Commission) are known to have increased bone density in the limbs, the mandible and the maxilla of the skull (B), especially around the roots of the tusks (Photo credit: Oliver Siddons at UCL Grant Museum of Zoology).

Bone microanatomy of pinnipeds shows similarities to both sirenians and cetaceans; some pinnipeds are pachyosteosclerotic while others are osteoporotic. Odobenids (walruses) are the only living representative of their Family (Figure 10A). Like other marine mammals, fossil walruses, such as Valenictus (Pliocene, 5.3-2.6mya), were also pachyosteosclerotic [43,44]. These large animals eat and dive at shallow depths, up to 100m, near the coast, and mainly feed on littoral foods such as clams and cockle [14,33,45]. Females and calves supplement their diet with worms and fish. Although walruses are clumsy in the terrestrial environment, they spend a considerable amount of time on land for resting, reproduction, and parturition and on pack ice near their food sources. Walruses can stand almost perpendicular to the sea floor with their heads down and their hind fins up. The ocean floor is disturbed with their vibrissae, forelimbs and possibly the tusks in order to unearth mollusks. Odobenids utilize a vacuum suction feeding technique that sucks mollusks into their mouth, separating the soft meaty parts from the shells [45-51]. In the case of walruses, pachyosteosclerosis in the mandible [51] and limbs [52] are associated with the unique body position and slow grazelike swimming while bottom feeding. Leverman et al. [50] also mentioned that the forelimbs are also used to excavate mollusks for the sea floor and causes the predominate limb bones to be larger than the other. Odobenids also have osteosclerotic maxillae to support their large tusks (Figure 10B). The tusks are used like a sledge while feeding [50] and sometimes used when climbing onto land or ice. Walruses are unique among pinnipeds because they have pharyngeal pouches that are used as floatation devices in the event of injury as well as for sleeping [45].
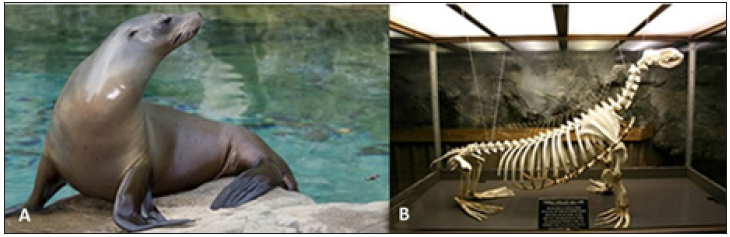
Wall [2] noted the high bone density of five otariid species that he studied, further supported by Nakijima & Endo [53]. However, neither author used the available terminology to describe which type of increased bone density these animals exhibit. Otariids (Figure 11) are known to use gastroliths as a mechanism against buoyancy much in the same way increased bone density is used for some other aquatic animals [17]. This anti-buoyancy mechanism is only employed by animals that utilize their forelimbs for swimming [3]. The literature on the Otariid (sea lions and fur seals) fossil record lacks histological or morphological evidence on bone density. Otariids are generally fast swimming hunters that can prey on pelagic fishes and benthic organisms. However, there are some species like the Cape fur seal (Arctocephalus pusillus) that typically hunt in the upper layers of the sea. Many otariids are opportunistic eaters that prey on other otariids, penguins, fish, crustaceans, and birds. They also haul themselves out of the water in order to give birth and rest [45]. Due to the variation in their diet and the use of gastroliths, bone microanatomy cannot be correlated to ecological niche in these animals.
Figure 11: Otariidae. (A) The California sea lion (Zalophus californianus; Photo credit: Smithsonian National Zoological Park) and the (B) skeleton (on display at Sea Lion Caves, Photo credit: Suzanne Phillips) of the modern Steller’s sea lion (Eumetopias jubatus; On display at Sea Lion Caves, Photo credit: Suzanne Phillips ). Otariids are generalist feeders that use gastroliths as a mechanism against buoyancy.
In Phocids (true seals) with pachyosteosclerosis, the increase in bone density corresponds with slower swimming speeds, shallow diving depth and prey availability as mentioned earlier. Unlike otariids, most true seals have specialized feeding mechanisms and habitats making a study of ecological niche and corresponding bone densities feasible. This also makes it possible to utilize bone density along with other ecomorphological characters to extrapolate possible feeding mechanisms and habitats in fossil seals.
Morphological analysis reveals that there are four subfamilies of phocids: Phocinae, Monachinae, Cystophorinae and Devinophocinae. The phocine Pusa hispida (ringed seal) has increased bone density and hunts slow moving prey in shallow water [14,19,33]. The Caspian (Pusa caspica) and the Baikal (Pusa sibirica) seals are closely related to the ringed seal but neither show any increased bone density [53]. The pachyostotic bones in some parts of the elephant seal (Mirounga leonina) skeleton (skull, mandible) have morphological significance (protection from fighting and feeding specializations) and are in contrast to the osteoporotic condition in some modern cetaceans [9,49]. The bone microanatomy of specific parts of the elephant seal skeleton must be examined in detail to decipher how these large-sized, sexually dimorphic, dietary specialized seals are able to dive deepest amongst all phocids, even with likely variation in bone density between the sexes. Male elephant seals are benthic feeders that dive deeper than their female counterparts. The females employ a different foraging mechanism by spending more time in the open waters and accessing the sea floor much less [54,55]. The females feed primarily on cephalopods in the mesopelagic portion of the ocean whereas males tend to stay on the continental shelf eating mollusks [56]. Fossil seals with known pachyosteosclerosis include two cystophorine species of the genus Pachyphoca who lived during the Middle Miocene (16-11.6 mya), consistent with the hypothesis that increase in bone density originated as a transitional phenotype in animals secondarily adapted to an aquatic lifestyle. Pachyphoca chapskii (Figure 12A & Figure 12B) and P. ukrainica both have primitive morphological characters that give them increased terrestrial locomotion than most modern representatives (such as large muscle attachments for both flexion and extension of the hip joint). However, only the fossil P. ukrainica (Figure 12C) and the modern male Cystophora cristata (Hooded seal) have a prominent lesser trochanter, as seen in terrestrial carnivores [19,56,]. The extinct subfamily Devinophocinae (Early Middle Miocene, 16.3- 14.9 mya) does not exhibit any increased bone density, suggesting that both described species (Devinophoca claytoni and D. emryi) were fast swimming and deep diving hunters [51,57,58]. Thus, further research is needed to determine if other members of the subfamilies Phocinae, Monachinae or Cystophorinae have variation in bone density that can be correlated to feeding preferences and environmental specializations.
Figure 12: Pachyphoca. Pachyosteosclerotic (A) sacrum and (B) humerus of the extinct cystophorine Pachyphoca chapski (Middle Miocene, Middle Sarmatian; ~12.3-11.2 mya).(C) Femur of Pachyphoca ukrainica showing the lesser trochanter (red box), which is associated with terrestrial locomotion (modified from Koretsky & Rahmat, 2013). The presence of pachyosteosclerosis, as well as characters associated with terrestrial locomotion, are found in animals in this extinct genus suggests that these seals were transitional species.

For many years, human evolution revolved around the “savannah” model credited to Dart [59], with apes leaving forest habitats for the savannah and humans evolving on the African savannah. It would have been on the savannah that the major step in human evolution, bipedalism, would have occurred largely due to the need for stealthy and fast hunting and predator evasion [59]. Napier [60] argued that a more diverse ecology could better explain human evolution, as bipedal movement would be advantageous for apes transitioning from the forest into grassland to visualize predators over the high grasses. More recently, Tobias [61] questioned the validity of the “savannah” model when several early bipedal humans were found with fossilized forest materials. Vaneechoutte, Kuliukas & Verhaegen [62] concluded that humans could not have evolved on the savannah and discussed the potential evolution of humans near and around water sources, ideas initially proposed by Serra in 1938 and Westenhöfer in 1942 [62].
In humans, increased bone density is usually associated with pathological conditions. The definitions for these conditions are slightly different than those associated with animals. Osteosclerosis can be defined as the calcification of cartilaginous tissue and seems to be associated with a pathological hindrance in endochondral ossification [15,62]. Munro [63] redefines human osteosclerosis, osteoporosis, pachyostosis, and other terms of bone density, explaining that historically they have all been associated with some pathological condition. Osteopetrosis (rock bone) is a group of pathologies that in humans leads to dense, brittle and calcium rich bones [62]. According to the Merck Manual, a resource for doctors and medical professionals on the latest in procedures, drugs, and diseases, osteosclerosis is a form of osteopetrosis that increases bone density without causing skeletal deformities [64]. Osteosclerosis is defined by Munro [63] as diffused or localized regions of hypermineralization due to pathological conditions such as sickle cell anemia and cancer. The definition by Gray et al. [15] associates a decreasing medullary cavity with osteopetrosis. Munro [63], however, treats medullary filling as a separate phenomenon from other bone states and refers to it as medullary stenosis. Hyperostosis is a general term for bone that is not in the proper place. Pachyostosis (broad bones) is a form of hyperostosis that is the result of periosteal ossification. Overall, the definition of human pachyosteosclerosis is comparable to that defined in animals because it combines the definitions of osteopetrosis, pachyostosis, and medullary stenosis by Munro [63] and Vaneechoutte, Kuliukas & Verhaegen [62].
The discovery of the early hominids, Peking man and Java man, first brought light to early humans having denser bones, with both noted to have thicker skulls. Weidenreich [65] tried to explain the correlation of thicker ancestral skulls to the present condition of humans by proposing the “Giant” theory, stating that all hominids descended from very large ancestors and skull thickness is related to body size. This theory has since been debunked as we know now that human body size has gone from smaller to larger over time. However, scientists have different theories as to why this cranial character has become a definable trait in the skulls of the modern human ancestor, Homo erectus [66].
Homo erectus (Figure 13A) has been shown to have higher bone density in the parietal bones of the skull, the femur, the tibia, and the ulna than their non-human primate counterparts. This increased density was originally considered pachyostosis [66], but recently has been reclassified as pachyosteosclerosis [62]. Copes & Kimbel [67] argue that the cranial vault thickness (Figure 13B) of Homo erectus is not unusual among other primates or hominids when using a residual analysis and after examining the brain capacity of each species, several species actually have higher residual values than Homo erectus. There is also a trend towards a decrease in trabecular density in the lower joints (ankles, knees, and hips; Figure 13C) of Homo over their life history, likely due to decreased mechanical load requirements due to the more stationary lifestyle of modern man when compared to the more nomadic lifestyle of early hominids [68]. Studies on Neanderthals (Homo neanderthalensis) and Homo sapiens demonstrated that the upper joints are not affected by decreasing bone density because the degree of which these joints are used has not changed [69].
Figure 13: Hominin. (A) An artist rendering of the face of Homo erectus (approx. 2 mya-100,000 ya; Photo credit: Smithsonian Museum of Natural History.) (B) Note the dense parietal bones in the skull (Modified from Boaz and Ciochon, 2004), recently associated with foraging in the water. (C) Pachyosteosclerosis has been identified in upper and lower limb bones as well (Photo credit: Smithsonian Museum of Natural History).

The Aquatic Ape Hypothesis (AAH) initially stated that early human adaptations (such as lack of hair or fur and decreased subcutaneous fat) were more suited for water environments than the savannah [62,70], further supported by recent evidence of the consumption of aquatic foods and bone microanatomy. An argument against AAH suggested that Homo erectus had a thicker cranial vault than modern humans, likely to help from collisions as early hominids hit each other on the head during fights and were forced to fight off/hunt large sized prey [63,66]. However, a counter argument suggests that in conflict, the thin frontal bone would still be vulnerable [63]. Also, a flattened, osteosclerotic skull would not serve a protective role because osteosclerosis causes bones to become brittle and flattened skulls are not as strong as rounded skulls [62]. Other researchers considered any human form of hyperostoses to be pathological and due to a lack of osteoclast activity [62].
Verhaegen & Munro [63] believe that pachyosteosclerosis in hominids, similar to what is seen in other taxa, is not pathological, but is due to heavy reliance on a semiaquatic lifestyle, supported by studies on bone microanatomy coupled with the discovery of sessile littoral organisms with H. erectus. Early humans would have lived in tribe groups that would have occupied different areas in the same ecosystem and, like macaques and baboons, would have been opportunistic omnivores [71]. Each tribe or group would have a slightly different dietary preference and would use different techniques to acquire resources based on availability. Therefore, some tribes or groups would have lived closer to water and became reliant on it as a food resource, developing techniques for harvesting littoral foods that may include a considerable amount of time in shallow waters. The discovery of evidence that more primitive hominin species, such as Neanderthals, used aquatic resources including dolphins, crocodiles, fish and seals in Gibraltar, Tanzania and Kenya further reinforces this hypothesis [72,15]. However, these discoveries do not mean that these early hominids were semi-aquatic or even aquatic. Early Homo ate the foods that were available to them, being opportunistic feeders that used tools [72-75].
Discussion
Researchers still debate the specific definitions of bone microanatomy terminology and the classification of increased bone density due to osteosclerosis or pachyostosis. The importance for new terminology to accurately describe what is observed has been acknowledged previously by others [3,76]. Some authors have tried to quantify the increases in bone density in order to make them less subjective, questioning what determines at which point does heavy bone become pachyostotic, osteosclerotic or pachyosteosclerotic [1,24]. There also should be better understanding of the mechanism in which the increased bone density has occurred, as osteosclerosis can be caused by incomplete endochondral ossification and/or inhibition of bone remodeling [39].
Bone microanatomy has been correlated to function and environment for many years [1-3,15,17,19,20,32,34,77]. In aquatic mammals, the degree of specialization for the water can be determined by bone density. This information can be used to extrapolate swimming and terrestrial locomotion, diving depths, aquatic habitat and possibly feeding behaviors. As mentioned above, the bone density seen in marine mammals is largely due to the ecological niche of each taxon. In taxa that are secondarily adapted to water, osteosclerosis, pachyostosis, and pachyosteosclerosis were transitional bone adaptations prior to the development of fins, flippers, or flukes. The resulting increase in density associated with these bone morphologies correlates with buoyancy control and/or stability in the water [1,15-17]. For large animals that employ a quadrupedal swimming mechanism and feed on mollusks, diving is an energetically expensive behavior. An increase in bone density is a hydrostatic mechanism that allows for bottom feeding with very little energy exertion.
Animals become best suited for their environment through the acquisition of beneficial traits. This could be accomplished either through the descent of advantageous traits (phylogeny) or through the development of traits in response to the environment (ecology). Phylogeny and ecology both contribute to bone microanatomy. However, which of these two factors gives the most significant contribution is a hotly debated topic. In the same bone, all the parameters that are not associated with compactness, such as the position of the transitional area between the medulla and the cortex, are known to have phylogenetic signal. Those characters that are most associated with compactness are correlated with ecology [35]. Moreover, within the same animal, certain bones have more phylogenetic signal than others. For instance, the weight bearing bones such as the tibia and those associated with food acquisition and processing such as the humerus tend to have a higher ecological signal than other bones because weight bearing bones are associated with locomotion and habitat whereas feeding behaviors are associated with food preference as well as habitat. And while microanatomy is mostly associated with ecological adaption, Kriloff, Germain & Laurin [35] suggest that osteological condition can be useful in phylogenetic analysis.
Increased bone density is an adaptation seen in many different taxa not closely related to one another. Therefore, in these taxa, this adaptation is likely a response to a change in environment and behaviors and not due to a shared lineage or close phylogenetic relationship [8]. The transition from land to shallow waters is where an increase in bone density would have been most advantageous, as these animals would eat slow moving fish and mollusks. Many marine mammals lost these higher density bones as an adaptation to new environments. The transition to deeper waters and faster moving prey makes a lighter, osteoporotic condition more advantageous [36]. Animals, such as some cetaceans and some pinnipeds, lost bone density over time, whereas other animals, such as sirenians, still retain pachyosteosclerosis. Both cetaceans and sirenians originated as terrestrial mammals that secondarily reinvaded the aquatic environment. As they became semi-aquatic, both groups increased bone density, acting as an energy efficient mechanism against buoyancy in animals that are bottom feeders with large lung volumes. These animals had legs and feet that are much less efficient for swimming than paddle-like fins, flippers and flukes [1,20]. Variation of bone density continued as animals became more specialized to the aquatic environment. Some cetaceans left the shallow waters and became deep diving, fast swimming, and fast prey catching animals. Sirenians, that have large lung volume, are able to remain in shallow environments, moving slow and eating sea grasses, as it is more energy efficient for them to have a hydrostatic mechanism (heavier bones) to negate buoyancy than a hydrodynamic mechanism (fin and fluke adjustment). The survey of taxa examined in this review demonstrates that changes in bone density occur in response to environmental and behavioral (i.e. feeding) conditions.
Similarly, placodonts and sauropterygians follow this same pattern [76]. In some described plesiosaurs from New Zealand such as pliosaurs, juveniles live and hunt in the shallows and have increased bone density. Adults lost this bone density and lived in the open waters as active predators [78]. Extinct sea snakes and all sirenians have increased bone density around the lungs in order to counteract the buoyancy of that region. It is expected that this trend would continue in pinnipeds. However, a thorough study has yet to be done.
Increased bone mass is usually present in the long bones, mandibles, vertebrae, skulls, and ribs. The increased density in the vertebrae is for buoyancy whereas the other bones (except the mandible and skull) have the added function to maintain the proper dorsal-ventral position. The mandible and skull appear to be both correlated to bottom feeding behaviors in the benthic zone whether it be diving with the head down or having large area for muscle attachments, advantageous for crushing shells. Therefore, pinnipeds that are bottom feeders in the shallow littoral zone should show increased bone density of the long bones, mandibles, vertebrae, skulls and ribs. For pinnipeds that live in the shallow waters but are not bottom feeders such as the ringed seal (Pusa hispida), it is reasonable to assume that these animals have increased density in long bones, vertebrae and ribs [79]. So far, we know that these animals have increased density in their humeri [19]. Deep divers of the pelagic zone that do not bottom feed should not show increased bone density and probably are osteoporotic. And for those that deep dive and feed on benthic fishes, they are expected to have at least some increased density in the mandible and skull. The extant harbor seal (Phoca vitulina) eats crustaceans and fishes that live in the shallow and coastal waters [80] and has increased bone mass in the ribs. Male southern elephant seals (Mirounga leonina) also have an increase in rib and mandibular bone density, but they are known deep divers and bottom feeders [81]. More studies have to be done to determine the density of other bones in all modern phocid taxa in order to confirm that bone density is the result of ecological niche/ feeding behaviors as seen in other marine mammals. This data can then be used to extrapolate possible ecological niche and feeding behaviors in extinct seal taxa.
It should also be noted that some taxa exhibit an unequal distribution of bone density. As mentioned earlier, placodonts have higher bone density in their humeri than in their femora. Bone density distribution can also be unequal in the same limb as the proximal bones of the sea otter (Enhydra lutris) have higher bone density than the distal bones (radius, ulna, tibia and fibula). These phenomena have been observed frequently but an explanation for the unequal distribution has yet to be rendered [9]. The chemical composition of bone should also change depending on the density of the bone. Therefore, the chemical composition of the bone would be different in shallow water seal species when compared to those that are deep divers. This might also be true even in closely related species, as any variation in bone composition would be due to different ecological niches.
Furthermore, previous studies have asserted that microanatomy of some bone can be used in phylogenetic analysis. The authors make clear that bone microanatomy has both ecological and phylogenetic contributions. However, bones that are most associated with locomotion and habitat such as the tibia and those associated in feeding behaviors such as the radius are more related to ecological niche and have less of a phylogenetic signal than other bones such as the fibula [35]. The effects of gravity would be lessened in an aquatic environment, with water acting as a better medium than air for bipedalism in animals that are traditionally quadrupeds. The decrease in trabecular density in the joints is due to a decrease in load requirements, possibly to facilitate wading in water. Because it is a convergent trait in different taxa, it would be safe to assume that a particular behavior or environment would facilitate its success. Yet, we don’t see other animals develop bipedalism when adapting to the aquatic environment. The popular hypothesis is the use of the forelimbs for foraging, carrying and eating [62]. The loss of quadrupedal locomotion in these taxa also leads to a reduction in the length of the forelimbs seen in modern humans. This affect is seen more dramatically in species such as the kangaroo and Tyrannosaurus rex. Opponents of this theory argue that human evolution progressed towards longer upper limbs, an increased upright position of the body, as well as increased bipedalism [70].
Thorpe, Holder & Crompton [82] disagreed with Langdon and supporters of the Aquatic Ape Hypothesis (AAH), stating that early hominids maintained long upper limbs while becoming more bipedal as a result of a woodland existence. If this is true, then humans may not have evolved from a quadrupedal ancestor similar to gorillas but maybe a tree dweller similar to an orangutan. Therefore, it is inferred that the upper limb has shortened over time [82]. The AAH remains controversial and has become popular again due to studies of bone microanatomy and the re-evaluation of previous evidence. Hominids are animals that evolution has molded into modern humans using the same pressures that other animals experienced [83,84]. It is not far reaching to think that there may have been an aquatic branch of the Homo family tree, similar to what is known in animals secondarily adapted to life in an aquatic environment. Therefore, the AAH must be evaluated further, as this hypothesis cannot be dismissed completely due to reasonable arguments and cannot be fully accepted due to the lack of evidence [85,86].
Conclusion
Osteoporosis is associated with fast swimming taxa that actively hunt fast moving prey. Increased bone density, whether through osteosclerosis, pachyostosis, or pachyosteoslcerosis, historically is associated with slow moving, shallow dwelling animals. This was true when the first terrestrial animals began to exploit aquatic food sources near the shore and is still true with modern aquatic and semi-aquatic species that collect sessile littoral foods. In semiaquatic animals, there is a positive correlation between the amount of time spent in water and the amount of increased bone density. This correlation, along with apparent convergent microanatomies, and the feeding and diving behaviors associated with those bone microanatomies, leads us to believe that bone density is more likely related to ecological and dietary preferences than to phylogenetic relationships.
Acknowledgement
We would like to thank Dr. Daryl Domning from Howard University (Washington, DC, USA), for his insight and helpful remarks. We would also like to thank Ms. Lindsey Koper and Ms. Azizah Hafed, members of the Laboratory of Evolutionary Biology, Howard University, for their encouragement. We also would like to thank the Smithsonian Museum of Natural History (Washington, DC, USA) especially Dr. Michael McGowen for access to their osteological collections.This article was funded by Torres Advanced Enterprise Solutions LLC.
Conflict of Interest
There are no conflicts of interest.
References
- Buffrénil VD, Canoville A, D'Anastasio R, Domning DP (2010) Evolution of sirenian pachyosteosclerosis: A model-case for the study of bone structure in marine Tetrapods. Journal of Mammalian Evolution 17(2): 101-120.
- Wall WP (1983) The correlation between high limb-bone density and aquatic habits in recent mammals. Journal of Paleontology 57(2): 197-207.
- Houssaye A (2009) Pachyostosis in aquatic amniotes: A review. Integrative Zoology 4(4): 325-340.
- Yan J, Clifton KB, Mecholsky JJ, Reep RL (2006a) Fracture toughness of manatee rib and bovine femur using a chevron-notched beam test. Journal of Biomechanics 39(6): 1066-1074.
- Yan J, Clifton KB, Reep RL, Mecholsky JJ (2006b) Application of fracture mechanics to failure in manatee rib bone. J Biomech Eng 128(3): 281-289.
- Buffrénil VD, Schoevaert D (1989) Quantitative data and histological observations on pachyostosis of the dugong skeleton, dugong dugon (Muller) (Sirenia, Dugongidae). Canadian Journal of Zoology 67(9): 2107-2119.
- Domning DP, Buffrénil VD (1991) Hydrostasis in the sirenia: Quantitative data and functional interpretations. Marine Mammal Science 7(4): 331-368.
- Buffrénil VD, Bardet N, Pereda-Suberbiola X, Bouya B (2008b) Specialization of bone structure in pachyvaranus crassispondylus Arambourg, 1952, an Aquatic Squamate from the late cretaceous of the Southern Tethyan Margin. Lethaia 41(1): 59-69.
- Houssaye A, Sander PM, Klein N (2016) Adaptive patterns in aquatic amniote bone microanatomy-more complex than previously thought. Integrative and Comparative Biology 56(6): 1349-1369.
- Kooyman GL (1973) Respiratory adaptations in marine mammals. American Zoologist 13(2): 457-468.
- Hua S, Buffrénil DV (1996) Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). Journal Vertebrate Paleontology 16(4): 703-717.
- Webb PW, Buffrénil VD (1990) Locomotion in the biology of large aquatic vertebrates. Transactions of the American Fisheries Society 119: 629-641.
- Scheffer VB (1976) A natural history of marine mammals. Charles Scribner's Sons, New York, USA, pp. 1-157.
- Reidman M (1990) The pinnipeds: Seals, sea lions and walruses. University of California Press, Los Angeles, USA, 5(11): 1-439.
- Gray N, Kainec K, Madar S, Tomko L, Wolfe S (2007) Sink or swim? Bone density as a mechanism for buoyancy control in early cetacean. The Anatomical Record 290(6): 638-653.
- Houssaye A, Tafforeau P, Muizon DC, Gingerich PD (2015b) Transition of Eocene whales from land to sea: Evidence of bone microstructure. PLOS One 10(2): e0118409.
- Taylor M (2000) Functional significance of bone ballastin in the evolution of buoyancy control strategies by aquatic Tetrapods. Historical Biology 14(1-2): 15-31.
- Houssaye A, Cornette R, Lee AH, Hutchinson JR (2015a) Biomechanical evolution of solid bone in large animals: a microanatomical investigation. Biological Journal of the Linnean Society 117(2): 350-371.
- Koretsky IA, Rahmat SJ (2017) Preliminary report on pachyosteosclerosis bone in seals. Open Access Research in Anatomy 1(1): 1-3.
- Reidenberg J (2007) Anatomical adaptations of aquatic mammals. The Anatomical Record 290(6): 507-513.
- Domning DP (2001) The earliest known fully quadrupedal sirenian. Nature 413: 625-627.
- Clementz MT, Goswami A, Gingerich PD, Koch PL (2006) Isotopic records from early whales and sea cow: Contrasting patterns of ecological transition. Journal of Vertebrate Paleontology 26(2): 355-370.
- Amson E, de Muizon C, Laurin M, Argot C, de Buffrénil V (2014) Gradual adaptation of bone structure to aquatic lifestyle in extinct sloths from Peru. Proceeding of the Royal Society B 281: 1-6.
- Canoville A, de Buffrénil V, Laurin M (2015) Microanatomical diversity of amniote ribs: An exploratory quantitative study. Biological Journal of the Linnean Society 118(4): 706-733.
- Buffrénil VD, Astibia H, Pereda-Suberbiola X, Berreteaga A, Bardet N (2008a) Variation in bone histology of middle Eocene sirenians from Western Europe. Geodiversitas 30(2): 425-432.
- Thewissen JGM, Williams EM, Roe LJ, Hussain ST (2001) Skeletons of terrestrial cetaceans and the relationship to artiodactyls. Nature 413: 277-281.
- Madar SI (2007) The postcranial skeleton of early Eocene pakicetid cetaceans. Journal of Paleontology 81(1): 176-200.
- Buffrénil VD, de Ricqlès A, Ray CE, Domning DP (1990) Bone histology of the ribs of the Archaeocetes (Mammalia: Cetacea). Journal of Vertebrate Paleontology 10(4): 455-466.
- Fish FE (1993) Influence of hydrodynamic design and propulsive mode on mammalian swimming energetics. Australian Journal of Zoology 42(1): 79-101.
- Buffrénil VD, Sire JY, Schoevaert D (1985) Comparison of the skeletal structure and volume between a delphinid and a terrestrial mammal. Canadian Journal of Zoology 64: 1750-1756.
- Buffrénil VD, Schoevaert D (1988) On how the periosteal bone of the delphinid humerus becomes cancellous: Ontogeny of a histological specialization. J Morphol 198(2): 149-164.
- Bedosti N, Landini W, Anastasio R (2012) The increase of bony mass in a small cyprinodontidae from the Messinian deposit of monte Tondo (Ravenna, Italy); Paleoecological implications. Proceedings of the Tuscan Society of Natural Sciences 122: 5-17.
- Heptner VG, Chapskii KK, Arseniev BA (1976) Mammalia of the Soviet Union pinnipeds and Cetacea. Moscow 2(3): 1-717.
- Fish FE, Stein BR (1991) Functional correlates of differences in bone density among terrestrial and aquatic genera in the family Mustelidae (Mammalia). Zoomorphology 110(6): 339-345.
- Germain D, Laurin M (2005) Microanatomy of the radius and lifestyle in amniotes (Vertebrata, Tetrapoda). Zoologica Scripta 34(4): 335-350.
- Cooper LN, Clementz MT, Usip S, Bajpai S, Hussain ST, et al. (2016) Aquatic habits of cetacean ancestors: Integrating bone micro anatomy and stable isotopes. Integrative and Comparative Biology 56(6): 1370-1384.
- Houssaye A (2013) Palaeoecological and morpho functional interpretation of bone mass increase: An example in late cretaceous shallow marine squamates. Biol Rev Camb Philos Soc 88(1): 117-139.
- Lee MSY, Caldwell MW, Scanlon JD (1999) A second primitive marine snake: Pachyophis woodwardi from the Cretaceous of Bosnia-Herzegovina. Journal of Zoology 248(4): 509-520.
- Houssaye A, Boistel R, Bohme W, Herrel A (2013) Jack-of-all-trades master of all? Snake vertebrae have a generalist inner organization. Naturwissenschaften 100(11): 997-1006.
- Northover J (2011) Skeletal morphology and evidence for swimming in a fossil stem pinniped, Puilla darwini, from the Canadian High Arctic. ProQuest Dissertations Publishing.
- Kruuk H (2006) Otters: ecology, behavior, and conservation. Oxford University Press, UK.
- Houssaye A, Botton DL (2018) From land to water: Evolutionary changes in long bone microanatomy of otters (Mammalia: Mustelidae). Biological Journal of the Linnean Society 125(2): 240-249.
- Deméré TA (1994) Two new species of fossil walruses (Pinnipedia: Odobenidae) from the upper Pliocene san Diego formation, California. Proceedings of the San Diego Society of Natural History 29: 77-98.
- Boessnecker RW (2017) A new early Pliocene record of the toothless walrus valenictus (carnivora, Odobenidae) from the Purisima formation of Northern California. Paleobios 34: 1-6.
- King JE (1983) Seals of the world. Cornell University Press, Ithaca, New York, USA.
- Fay FH (1982) Ecology and biology of the pacific walrus, Odobenus rosmarus divergens North American Fauna 74: 1-279.
- Kastelein RA, Mosterd P (1989) The excavation technique for mollusks of pacific walruses (Odobenus rosmarus divergens) under controlled conditions. Aquatic Mammals 15(1): 3-5.
- Kastelein RA, Wiepkema PR, Slegtenhort C (1989) The use of mollusks to occupy pacific walruses (Odobenus rosmarus divergens) in Human Care. Aquatic Mammals 15(1): 6-8.
- Koretsky IA, Rahmat SJ, Peters N (2014) Remarks on correlations of implications of the mandibular structure and diet in some seals (Mammalia, Phocidae). Vestnik Zoologii 48(3): 255-268.
- Levermann N, Galatius A, Ehlme G, Rysgaard S, Born EW (2003) Feeding behavior of free-ranging walruses with notes on destrality of flipper use. BMC Ecology 3(9).
- Rahmat SJ, Koretsky IA (2015) Diversity of mandibular morphology in some carnivorans. Vestnik Zoologii 49(3): 267-284.
- Maas M (2009) Histology of bones and teeth. In: Perrin WF, Wursig B, Thewissen JGM (Eds.), Encyclopedia of marine mammals (2nd edn), Academic Press, New York, USA, pp. 124-129.
- Nakajima Y, Endo H (2013) Comparative humeral microanatomy of terrestrial, semiaquatic, and aquatic carnivorans using microfocus CT Scan. Mammal Study 38(1): 1-8.
- Hindell MA, Slip D, Burton HR (1991) The diving behavior of adult male and female southern elephant seals, Mirounga leonine (Pinnipedia: Phocidae). Australian Journal of Zoology 39(5): 499-508.
- Lewis R, O'Connell TC, Lewis M, Campagna C, Hoelzel AR (2006) Sex-specific foraging strategies and resource partitioning in the southern elephant seal (Mirounga leonina). Proceedings of the Royal Society B 273(1603): 2901-2907.
- Koretsky IA, Rahmat SJ (2013) First record of fossil cystophorine (Carnivora, Phocidae): Middle Miocene seals from the Northern Paratethys. Rivista Italian di paleontological e stratigraphic 119(3): 325-350.
- Koretsky IA, Holec (2002) A primitive seal (Mammalia: Phocidae) from the early middle Miocene of central Para Tethys. In: Emry RJ (Ed.), Cenozoic mammals of land and sea: Tributes to the career of clayton E. ray. Smithsonian Contributions to Paleobiology 93: 163-178.
- Rahmat SJ, Koretsky IA (2016) First record of postcranial bones in devinophoca emryi (Carnivora, Phocidae, Devinophocinae). Vestnik Zoologii 50(1): 71-84.
- Dart RA (1925) Australopithecus africanus-the man-ape of South African. Nature 115: 195-199.
- Napier J (1967) The Antiquity of human walking.
- Tobias P (2011) Revisiting water and hominin evolution. In: Vaneechoutte M, Kuliukas A, Verhaegen M (Eds.), Was man more aquatic in the past? Fifty years after Allister hardy-waterside hypothes in human evolution. Bentham Science pp. 3-15.
- Vaneechoutte M, Kuliukas A, Verhaegen M (2011) Was man more aquatic in the past? Fifty years after Allister hardy-waterside hypotheses of human evolution. Bentham Science pp. 1-254.
- Munro S, Verhaegen M (2011) Pachyosteosclerosis in archaic homo: Heavy skulls for diving, heavy legs for wading. In: Vaneechoutte M, Kuliukas A, Verhaegen M (Eds.), Was man more aquatic in the past? Fifty years after Allister hardy-waterside hypotheses of human evolution. Bentham Science, UAE.
- Pessler F (2019) Overview of osteopetrosis (Marble Bone). Merck manual professional version.
- Weidenreich F (1946) Apes, giants and man. University of Chicago Press, Chicago, USA, pp. 1-128.
- Boaz NT, Ciochon RL (2004) Dragon bone hill: An ice-age saga of homo erectus. Oxford Press, New York, USA.
- Copes LE, Kimbel WH (2016) Cranial vault thickness in primates: Homo erectus does not have uniquely thick vault bones. J Hum Evol 90: 120-134.
- Chirchir H, Kivell TL, Ruff CB, Hublin J, Carlson KJ, et al. (2015) Recent origin of low trabecular bone density in modern human. Proceeding of the National Academy of Sciences of the United States of America 112(2): 366-371.
- Ryan TM, Shaw CN (2015) Gracility of modern homo sapiens skeleton is the result of decreased biomechanical loading. Proceedings of the National Academy of Sciences of the United States of America 112(2): 372-377.
- Langdon JH (1997) Umbrella hypothesis and parsimony in human evolution: A critique of the aquatic ape hypothesis. J Hum Evol 33(4): 479-494.
- Elton S (2006) Forty years on and still going strong: The use of hominin-cercopithecid comparisons in paleoanthropology. Journal of the Royal Anthropological Institute 12(1): 19-38.
- Stewart KM (1994) Early hominid utilization of fish resources and implications for seasonality and behavior. Journal of Human Evolution 27(1-3): 229-245.
- Shipman P (2008) Separating "us" from "them": Neanderthal and modern human behavior. Proceedings of the National Academy of Sciences of the United States of America 105(38): 14241-14242.
- Stringer CB, Finlayson JC, Barton RNE, Fernandez JY, Caceres I, et al. (2008) Neanderthal exploitation of marine mammals in Gibraltar. Proceedings of the National Academy of Sciences of the United States of America 105(38): 14319-14324.
- Braun DR, Harris JW, Levin NE, McCoy JT, Herries AI, et al. (2010) Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proceedings of the National Academy of Sciences of the United States of America 107(22): 10002-10007.
- Klein N, Sander PM, Krahl A, Scheyer TM, Houssaye, A (2016) Diverse aquatic adaptations in Nothosaurus (Sauroptergia)-Inferences from humeral histology and microanatomy. PLOS One, USA.
- Laurin M, Canoville A, Germain D (2011) Bone microanatomy and lifestyle: A descriptive approach. Comptes Rendus Palevol 10(5-6): 381-402.
- Wiffen J, Buffrénil VD, de Ricqles A, Mazin J (1995) Ontogenetic evolution of bone structure in late cretaceous Plesiosauria from New Zealand. Geobios 28(5): 625-640.
- Nachtsheim DA, Jerosch K, Hagen W, Plotz J, Bornemann H (2017) Habitat modelling of crabeater seals (lobodon carcinophaga) in the Weddell sea using the multivariate approach maxent. Polar Biology 40(5): 961-976.
- Bigg MA (1981) Harbour seal-phoca vitulina and Largha P. In: Ridgway SH, Harrison RJ (Eds.), Handbook of marine mammals. Academic Press, New York, USA.
- Ling JK, Bryden MM (1981) Southern elephant seal. In: Ridgway SH, Harrison RJ (Eds.), Handbook of Marine Mammal. Academic Press, New York, USA.
- Thorpe SK, Holder RL, Crompton RH (2007) Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science 316(5829): 1328-1331.
- Dion N, Ste-Marie LG (2012) The fragile beauty of bone architecture. Medicographia 34: 163-169.
- Houssaye A, Mazurier A, Herrel A, Volpato V, Tafforeau P, et al. (2010) Vertebral microanatomy in squamates: Structure, growth and ecological correlates. Journal of Anatomy 217(6): 715-727.
- Kierszenbaum AL, Tres LL (2012) Histology and cell biology: An introduction to pathology. (3rd edn), Elsevier Saunders, Philadelphia, USA.
- Verhaegen M, Munro S (2011) Pachyosteosclerosis suggests homo frequently collected sessile littoral foods. Journal of Comparative Human Biology 62(4): 237-247.
© 2020 Madelyn Crowell. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






