- Submissions

Full Text
Open Access Biostatistics & Bioinformatics
COVID-19 Epidemic: China and ex-China
David N, Hwang L, Cynthia L, Vuong N Trieu*
Virology Program, Mateon Therapeutics Inc., Agoura Hills, CA
*Corresponding author: Vuong N Trieu, Virology Program, Mateon Therapeutics Inc., Agoura Hills, CA
Submission: April 09, 2020;Published: April 20, 2020

ISSN: 2578-0247 Volume3 Issue1
Abstract
Here we report the current COVID-19 epidemic in terms of mortality and recovery compared to the confirmed patient population of provinces in China as well as countries outside of China. Data was obtained from the Center for System Science and Engineering (CSSE) by Johns Hopkins University (JHU) and was plotted in log-log charts. For China, mortality dropped as low as 0.08% but then converged to a band of 1%-5%, with the median value of 1.1% as of March 9th, 2020. For countries outside of China, mortality dropped to a low of 0.2% with a median value of 2.4% as of March 9th, 2020. This difference was statistically significant with p=0.0057.A bi-modal distribution in mortality was observed for both China and countries outside of China, which would concur with reports mentioning two possible strains of the SARS-CoV-2 virus. China exhibited a median recovery rate of 95.0% with the lowest being Hubei province with a recovery rate of 57%. Outside of China, the median recovery rate was 10.7% and was significantly lower than that of China, p <0.0001, t-test. Distribution-wise, both China and countries outside of China were observed to be similar. As of now, the spread of COVID-19 in countries outside of China are showing properties more similar to that of Hubei–the epicenter of COVID-19 epidemic in China.
Keywords COVID-19; SARS-CoV-2; Coronavirus; Epidemic
Introduction
Coronaviruses make up a large family of viruses that can infect birds and mammals, including human, and have been responsible for several outbreaks around the world, including the severe acute respiratory syndrome (SARS-CoV), the Middle East respiratory syndrome (MERS-CoV), and the most recent novel coronavirus (COVID-19). Belong to family Coronaviridae, these are enveloped, positive-stranded viruses with ~30,000 nucleotides [1]. They are broadly divided into three groups: 1) Transmissible gastroenteritis coronavirus (TGEV), porcine gastroenteritis virus etc.; 2) SARS-CoV, mouse hepatitis virus (MHV) etc.; and 3) avian infectious bronchitis virus (AIBV) etc. [2]. Since late 2019, an outbreak of upper respiratory infection and pneumonia caused by a novel coronavirus (COVID-19) has rapidly spread from its epicenter in Wuhan of Hubei province to become a global epidemic with hundreds of thousands of cases and thousands of deaths. It is believed the outbreak has a zoonotic origin with an animal to human transmission followed by a human to human spread via aerosol droplets and contaminated surfaces. As with the prior outbreaks of SARS and MERS, numerous approaches are being taken in an attempt to treat and prevent the disease. The genome information for COVID-19 is known and has been shared. A reliable assay using real-time reverse transcription-polymerase chain reaction (RT-PCR) has been developed and is in widespread use. There are reportedly over 100 clinical studies in progress in China alone targeting the diagnosis and treatment of COVID-19. On the clinicaltrials.gov website, there are at least 50 such clinical studies recorded and the list is growing each day. Most are active and enrolling patients. These trials span the therapeutic spectrum and include some diagnostic studies and some trials evaluating traditional medicine and herbal remedies. Most of the listed studies are assessing existing drugs with some evidence of antiviral activity either as monotherapy or in combination. The classes of agents include antivirals (protease inhibitors, nucleotide analogs), non-steroidal anti-inflammatory drugs, corticosteroids, immunomodulators, monoclonal antibodies, polyclonal antibody preparations, washed microbiota and umbilical cord mesenchymal stem cells.
The preponderance of studies and the most robust ones are evaluating antiviral agents such as remdesivir, lopinavir, ritonavir, oseltamivir, sofosbuvir, and ribavirin, often in combination. Most of these agents had been used during the SARS and MERS outbreaks. Randomized studies, however, were generally not able to be performed and except for anecdotal evidence of their therapeutic effect, none of these drugs have been approved for the treatment of any coronavirus illness. It remains to be seen whether they will demonstrate clinically meaningful efficacy against COVID-19. There is a clear need for new drugs both preventive (vaccines) and therapeutic. One new approach being pursued by some groups is antisense oligonucleotides which would interfere with RNA synthesis and viral replication. Coronavirus has a relatively large genome of approximately 27 to 34 kilobases which offers multiple sequences for antisense therapy. Antisense technology is well-suited to address a COVID-19 outbreak. Antisense drugs work at the molecular level by binding to messenger RNA to interrupt the process by which disease-related proteins are produced. These drugs are highly selective and able to target areas in RNA that less likely to mutate. The pharmacology antisense oligonucleotide agents are very different than antivirals of the protease inhibitor or nucleotide analog classes and there is a possibility that combination therapy using different pharmacologic mechanisms may prove to be a more effective approach against COVID-19. Antisense oligonucleotide targeting the SARS genomic sequence which is very similar to the COVID-19 is under development by Mateon Therapeutics and is expected to be available for clinical testing shortly. To facilitate the development of these therapeutics, we compare and contrast the COVID-19 epidemic in China and ex-China. The analyses are reported here.
Results and Discussion
Mortality rate
Log-log plots of mortality versus confirmed cases and recovered rate versus confirmed casesof China and ex-China were performed to compare the COVID-19 epidemic in China versus ex-China. Rate has a negative power relationship to the number of confirmed cases, probably because with the initial mortality, aggressive contact tracing and diagnostic testing, confirmed cases increased causing rate to drop. For China, mortality dropped as low as 0.08% but then converged to a band of 1%-5%, with the 3-09-2020 median value of 1.1% (0.08, 4.4, N=27), median (min, max, N=provinces). Hubei, the epicenter of the epidemic, maintained a high median mortality rate of 3.5% (2.7, 5.3, N=48), median (min, max, N= number of readings). Mortality and confirmed cases exhibited a negative power relationship described by the following equation: Mortality=-0.0126 × Confirmed Cases-0.8916 (Figure 1).
Figure 1:
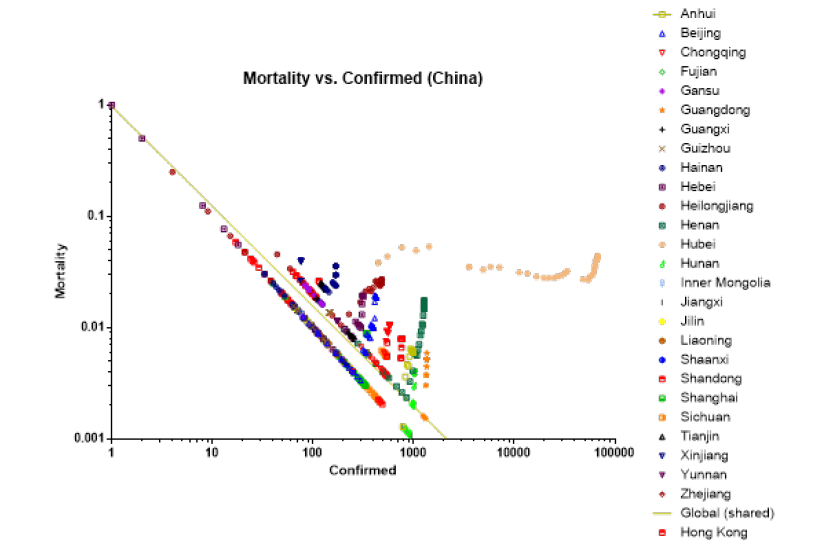
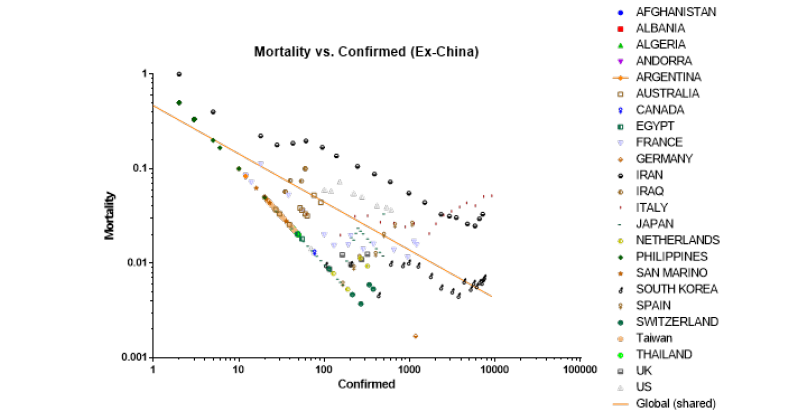
For ex-China, mortality dropped to a low of 0.2% with median value of 2.4% (0.2, 10.0, N= 20), median (min, max, N= countries). This was statistically significantly higher than China, p=0.0057, t-test. Mortality and confirmed cases exhibited a negative power relationship described by the following equation: Mortality=-0.3332 × Confirmed Cases -0.5092 (Figure 2). The slope for this equation was only 57% of that of China.
Figure 2:
Recovery rate
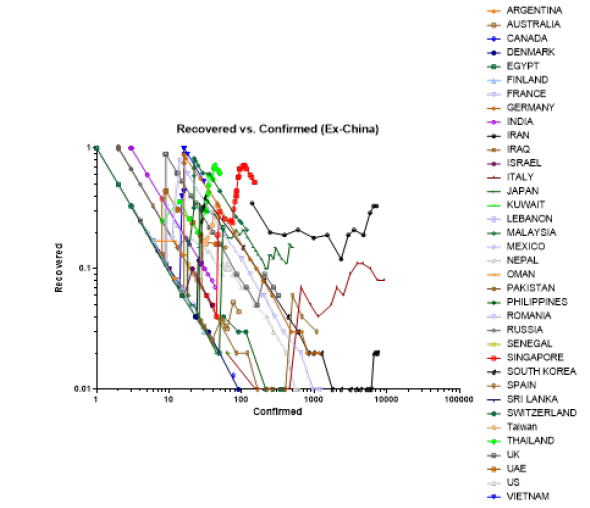
China exhibited a median recovery rate of 95.0% (57, 100, N= 33), median (min, max, N=provinces) with the lowest being Hubei with a median recovery rate of 57% (Figure 3). Ex-China recovery was significantly lower than that of China, p<0.0001, t-test. Ex-China median recovery rate was 10.7% (1.0, 100, N=36), median (min, max, N= countries) (Figure 4). The high median recovery rate for China was consistent with the epidemic being under controlled in China whereas the median recovery rate of ex-China countries was more consistent with the beginning of the epidemic. Strikingly, China was able to control the epidemic at 100-1000 confirmed cases, except for Hubei where the epidemic remained out of control until 10,000-100,000 confirmed cases. Ex-China countries were unable to control their epidemic at the 100-1000 mark.
Figure 3:
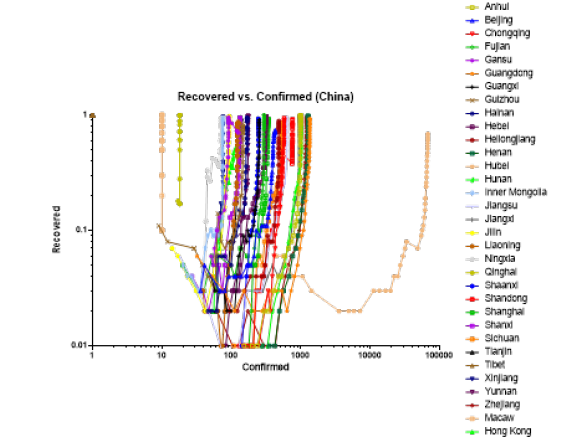
Figure 4:
Comparison of China vs. ex-China
Mortality exhibited bi-modal distribution for both China and ex-China with the lower mortality node being less prominent ex-China (Figure 5). This would be consistent with the two strains hypothesis recently suggested [3] with one strain being more infective and possibly less lethal. As for recovery, ex-China and China are similar in frequency of distribution of recovery (Figure 6).
Figure 5:
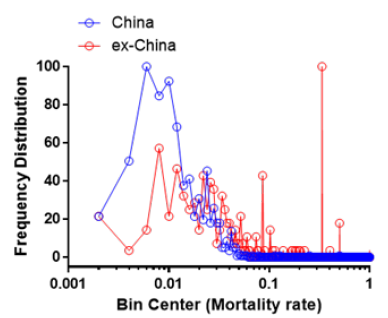
Figure 6:
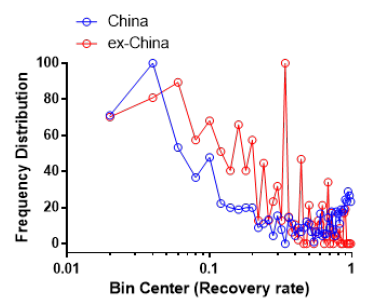
Conclusion
This study was performed to understand the spread of COVID-19 epidemic outside of China.Given the higher mortality rate than China and the failure to control the epidemic at the same 100-1000 mark as China, the COVID-19 epidemic outside of China is expected to be possibly be as severe as that of Hubei- the epicenter of the COVID-19 epidemic. It is noteworthy that the mortality curves and recovered curves are more similar to Hubei for ex-China. Of particular concern is the slow rise in recovery rate- even in Korea where the epidemic is being managed effectively. The differences between the China and ex-China are either containment/control and/or treatment. Containment and control such as quarantine and social distancing are more draconian in China and ex-China may not be able to implement especially when the epidemic has passed the 100-1000 confirmed cases. Therapeutic interventions such as those explored in China should be examined and implemented to slow down the spread of COVID-19 in addition to containment and control. Among the potential therapeutics the Gilead drug is promising but may have undesirable toxicities [4]. Other therapeutics should be explored quickly in order to contain the epidemic. Among the approaches that should be examined is the antisense oligotherapeutics approach due to its rapid first in man pathway- Milasen an oligo therapeutic was able to enter first in man testing with just a 30 day rat tox study [5]. We have developed several oligotherapeutics that could piggy back on this rapid clinical path.
It may be possible that COVID-19 will become endemic due to community spread observed in the US and other countries. Once endemic, the risk of the epidemic re-emerged in areas previously controlled areas such China. Once COVID-19 is endemic, the therapeutics will be invaluable; however, a vaccine would be necessary for mass immunization. Vaccine against corona virus does have some technical challenges- vaccine against the S-antigen of SARS has liver toxicities which prevented its further development [6]. The development of vaccine has been accelerated recently with RNA/DNA based vaccines; however, the side effects of these vaccines need to be studied rigorously to avoid unexpected side effects that magnified with mass vaccination.
Materials and Methods
COVID-19 data (daily confirmed, mortality, and recovery) was obtained utilizing the GIS Dashboard prepared by the Center for System Science and Engineering (CSSE) by Johns Hopkins University (JHU). JHU CSSE provides a visualized form of data collected daily starting from January 22, 2020 (February 1, 2020, for the USA) with the primary data source coming from DXY, an online platform provided by the Chinese medical community and is bolstered by social media posts, online news services, and direct communications sent through the dashboard. Confirmation is made directly with local and regional health departments [7]. Confirmed, mortality, and recovery data were collected between the dates of January 22, 2020, and March 9, 2020. The data was provided in CSV format at a Github repository provided by the JHU CSSE and plotted utilizing GraphPad Prism 6.07 (GraphPad Software, San Diego, California). To provide a more consistent view, number of confirmed patients, deaths, and recoveries in states/provinces of countries outside of China, i.e. USA, Canada, and Australia, were combined.
References
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R et al. (2003) Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300(5624): 1394-1399.
- Spaan WJM, Cavanagh D (2004) Coronaviridae, in Virus Taxonomy, VIIIth Report of the ICTV. Elsevier Academic Press London, UK 945-962.
- Tang X, Wu C, Li X, Song Y, Yao X et al. (2020) On the origin and continuing evolution of SARS-CoV-2. National Science Review.
- Midgley CM (2020) First 12 patients with corona virus disease 2019 (COVID-19) in the United States. medRxiv.
- Kim J, Hu C, Moufawad El AC, Black LE, Douville J (2019) Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med 381(17): 1644-1652.
- Du L, He Y, Zhou Y, et al. (2009) The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol 7(3): 226-236.
- Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 Feb 19.
© 2020 Vuong N Trieu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






