- Submissions

Full Text
Open Access Biostatistics & Bioinformatics
Detection of protein complexes in blood plasma synthesized by malignant glioma cells and their microenvironment by dynamic light scattering depending on the degree of the blood-brain barrier permeability
Gridina NYa*1, Chunikhin AYu2, Morozov AN1, Rozumenko VD1, Draguntsova NG1, Belousova AD1, Tarasov AL3, Beloshitsky VV1
1Institute of Neurosurgery n A P Romodanov NAMSU, Kiev, Ukraine
2Institute of Biochemistry n A V Palladin NASU, Kiev, Ukraine
3Institute of Cybernetics n V M Glushkov NASU, Kiev, Ukraine
*Corresponding author: Gridina N Ya, Institute of Neurosurgery n. A. P. Romodanov NAMSU, Kiev, Ukraine
Submission: April 03, 2020;Published: April 17, 2020

ISSN: 2578-0247 Volume3 Issue1
Abstract
In the blood plasma of patients with malignant gliomas of the brain by method dynamic light scattering protein complexes with diameter of more than 150nm were detected, indicating increased permeability of the blood brain/tumor (BBTB) and blood-brain (BBB) barriers. The results of these studies are taken as the basis for the development of the express method for determining the increased permeability of the BBTB and BBB in neurosurgical patients. The method can be used at a certain stage of chemotherapy for patients with malignant gliomas of the brain, depending on the permeability of the BBTB and BBB.
Keywords: Dynamic light scattering method; Malignant gliomas of the brain; BBB; BBTB; Permeability; Express method
Introduction
The method of dynamic light scattering (DLS) has some advantages over classical methods for the determination of proteins and their complexes in serum and plasma, especially when it is necessary to determine the characteristics of a large number of these biologically active compounds. The range of sizes of molecular particles that can be determined by this method includes protein, viral, nucleotide, and other sources of interest in various fields of science, starting with physics and ending with the latest research in biotechnology [1-12]. Of particular interest are studies by the DLS method in the field of oncology, which makes it possible to diagnose in oncology and inflammatory diseases, determine the severity of patients in urgent conditions, etc. [13-16]. Studying the features of the tumor microenvironment showed that tumor-infiltrating cells can release a wide variety of growth factors, cytokines/chemokines, and enzymes that support tumor growth, angiogenesis and invasion processes [17]. In addition to microglia and macrophages of the brain, glioma cells are also infiltrated by peripheral blood cells of inflammatory origin, including lymphocytes and monocytes [18]. An adaptive immune system in tumors can exhibit both protective functions and support a chronic inflammatory process in tumor microenvironment, which promotes tumor progression. The mechanisms of the protein synthesizing function of blood cells in stage I of tumor associated inflammation (TAI) are poorly understood, although they are of great interest in the pathogenesis of glioma progression. Using the DLS method, it is possible to investigate some features of the appearance of protein complexes (PC) in the blood plasma with a certain hydrodynamic diameter during inflammation associated with the growth of malignant gliomas of the brain. Indeed, for a long time it was believed that there are no inflammatory processes in the brain, because the brain is protected by the blood-brain barrier (BBB) [19]. Depending on the degree of BBB permeability, detection of PC in the blood plasma of patients synthesized by tumor microenvironment glioblast cells is possible. A study of these PCs will allow their synthesis to be associated with the activation of TAI and the subsequent progression of gliomas to glioblastomas and death of patients. On the other hand, the determination of PC of a certain size in the blood plasma of patients will make it possible to judge the degree of BBB openness and prescribe drugs based on this knowledge.
Objective
To compare the sizes of PCs in the blood plasma of patients with glioblastomas with the sizes of PCs, released during in vitro cultivation of tissue fragments with glioblastomas, removed during surgical interventions. Development of an express method for determining the degree of BBB and BBTB permeability by dynamic light scattering
Materials and Research Methods
Patients before starting treatment on an empty stomach took 10 ml of blood from the ulnar vein. After centrifugation at 1500rpm/10min plasma and blood cells were separated. Then plasma was centrifuged for 10 minutes at 9 thousand rpm/10 minutes. The volume of one plasma sample for measuring by the DLS method on a laser correlation spectrometer was 1.5ml [20]. The measurements were performed three times. Protein complexes (PC) were studied in 60 patients with malignant gliomas of the brain and spinal hernias. The growth of malignant gliomas is associated with aseptic microinflammation, and spinal hernias contribute to the development of non-tumor inflammation. The tumor tissues were cultured with glioblastoma, taken during surgical interventions in 3 patients in RPMI medium without serum supplemented with antibiotics. After 72 hours, the supernatant, containing protein complexes (PC) isolated by tumor cells was centrifuged at 9,000 rpm/10 minutes. The size distributions of blood plasma PCs of patients with glioblastomas were compared with the size distributions of PCs, isolated by tumor tissues into RPMI nutrient medium. If the sizes of PCs from glioblastoma cells coincide with the sizes of PCs that appear in the blood plasma spectrum of patients with malignant gliomas, it can be assumed that PCs penetrate into the blood plasma as a result of increased permeability of the BBB and BBTB [21]. Such an assumption should be proved experimentally. The size of protein complexes was studied by dynamic light scattering using a ZetaSizer-3 laser correlation spectrometer (Malvern Instruments, UK) equipped with a LGN-111 He-Ne laser (P=25 mW, ƛ=633 nm). The measuring range of the device is from 1nm to 20 microns. The measurement principle is based on the analysis of the correlation characteristics of fluctuations in the intensity of scattered light when a laser beam passes through a dispersed medium. Measurement of the correlation function of fluctuations in the intensity of scattered light and the integral intensity of scattering allows us to determine the coefficient of translational diffusion of dispersed particles in liquids and to calculate the distribution of nanoparticles by hydrodynamic diameter using the Stokes-Einstein equation. The translational diffusion coefficient D of particles is related to the correlation time Ʈ with the relation:
Dq2=1/τc (2.1)
The wave vector of concentration fluctuations q is described by the expression:
q=(4πn/λ0) sin (Θ/2) (2.2),
where n is the refractive index of the medium (liquid),
λ0 is the radiation wavelength,
Θ is the scattering angle.
Using the Stokes Einstein formula, which relates the particle size to their translational diffusion coefficient and fluid viscosity (culture medium), we determine the hydrodynamic diameter of the protein spherical particles:
d (H)=kBT/3πηD (2.3),
where kB is the Boltzmann constant,
T is the absolute temperature,
η is the shear viscosity of the medium in which the PC are weighed,
D is the translational diffusion coefficient.
Registration and statistical processing of indicators of laser radiation scattered from an aqueous (n 1.33) suspension of protein complexes was performed three times for 60 sec. at a temperature of +220C at a scattering angle of 900 [22-26]. Statistical processing of the obtained data was carried out in the “Statistica-5.5” package using non-parametric data estimation methods. We evaluated the correct distribution of the characteristics for each of the obtained variation series, the average values for each characteristic that were studied, and standard deviations. The significance of the difference between the independent quantitative values, using the U-criterion of Mann-Whitney, and between the dependent ones using the Wilcoxon criterion.
Research Results and Discussion
To solve the problem, studies were conducted using the method of dynamic light scattering [22,26] of blood plasma in patients with brain gliomas and spinal hernias. The DLS method has been successfully used to study the sub fractional composition of various biological fluids [22,27]. For monodisperse systems, the DLS method allows one to determine with high accuracy the diffusion coefficients of particles and calculate their hydrodynamic diameter. As applied to serum/plasma, 5 discrete zones are proposed depending on the diameter of the light scattering particles: I (0-10 nm); II (11-30 nm); III (31-70 nm); IV (71-150nm); V (above 150nm), etc. The choice of zones was carried out empirically on the basis of studying the nature of multiparameter shifts in the serum homeostasis system of more than 10 no sological forms studied in various medical institutions when testing the DLS method, as well as when experimentally modeling some pathological processes [22]. In studies, the program of analysis of the redistribution of parameters of protein complexes was first used [28]. This program is an automated system for finding in the blood plasma the differential-significant zones of PC among various types of pathologies. The data for this system are the number of PCs with certain diameters and the volume occupied by molecules with a given diameter in a certain size range. With its help, new discrete zones were found, with maximum efficiency, showing differences in changes in protein complexes depending on the type of neurosurgical pathology. The polydispersity index in the samples did not exceed 0.4. Protein complexes with diameters ranging from 1 to 10nm occupy the maximum volume in blood plasma over 80% in healthy individuals (Figure 1).
Figure 1: The ratio of CD volumes with diameters in the range from 1 to 1500nm in the plasma of healthy individuals and RPMI nutrient medium in which tissues were cultured with glioblastoma tissue.

The fact of the practical absence of CD with diameters from 1 to 150nm in the RPMI nutrient medium in which tissues were cultured with glioblast was noted, which may indicate their presence exclusively in the blood plasma of patients. In the I zone (0-10nm) mainly low molecular weight monomeric proteins and their hydrolysis products fall; in the II zone (11-30nm) globulins and low molecular weight lipoprotein complexes; in III (31-70nm) higher molecular weight glycoprotein complexes, ribonucleic and deoxyribonucleic particles, as well as the lowest molecular weight immune complexes; in the IV zone (71-150nm), medium-sized immune complexes predominate, in the V zone (150-360nm) large cyclic immune complexes (CICs) predominate, in the VI zone (360-2000nm) aggregates of various proteins are determined. The V zone (150-360 nm) is filled when immunopoiesis is induced in the body with the formation of high molecular weight immune complexes, which are most often associated with allergization and autoimmune sensitization [22]. The maximum volume over 70% was occupied by PCs with diameters ranging from 150 to 360nm, secreted into the growth medium by glioblastoma cells (Figure 1). The data obtained using the DLS method indicate a variety of sizes of PCs, their number in the sample volume, both in blood plasma and in the cultivation of blood cells taken from healthy and patients with inflammation (spinal hernias) and malignant gliomas of the brain. The maximum volume in blood plasma is occupied by PC with a diameter in the range from 1 to 10nm. PC of tumor origin occupy a maximum volume in the diameter range from 150nm and above. An analysis of the ratio of volume distributions of PC in blood plasma allows us to judge the degree of permeability of the BBB and BBTB in malignant gliomas.
Determination of the permeability of the blood-brain/tumor barriers for malignant gliomas
We studied the volume distributions of the hydrodynamic diameter of blood plasma PC in groups of healthy donors (11 patients), patients with spinal hernias, and patients with malignant gliomas of the IV degree of malignancy (27 patients before and 22 patients after operation). To compare the volume distributions of protein complexes of tumor origin, human glioblastoma tissues were cultured. A comparative analysis of the volume distributions of protein structures isolated by tumor tissue fragments of 0.2-0.3 mm3 in volume was carried out when they were cultured for 72 hours in RPMI medium with volume distributions of protein structures of blood plasma before and after surgery. The hydrodynamic diameters of PCs in the measured blood plasma volumes in healthy and patients with spinal hernias were found in the range from 5 to 100 nm, taking into account individual fluctuations. In the cultured liquids, additional hydrodynamic diameters of PC ranging from 150nm and higher, synthesized by glioblastoma tissues, were found. In the blood plasma of patients with glioblastomas on the 7th day after the operation, additional hydrodynamic diameters of PC were also found in the same range as in the tumor tissues. With an increase in the permeability of BBTB in the blood plasma of patients, additional hydrodynamic diameters of PCs exceeding 150nm appear, identified as high molecular weight circulating immune complexes (CICs) and hydrodynamic diameters of protein aggregates in the range from 100 to 2000nm. It should be noted that increased of BBTB permeability in glioblastomas was observed only in 6 patients out of 27 before the operation, and in 6 patients out of 22 on the 7th day after the operation.
In patient A, the number of CDs with sizes over 150nm was greater than that of patient M, before surgery and significantly more on the 8th day after surgery (Figures 2&3). In patient M., before and after surgery, PC in the blood plasma is determined (Figures 4&5), however, in a significantly smaller amount than in patient A. Patient Sh. is not determined before PC surgery with sizes larger than 150nm , which gives grounds to consider the BBTB closed . But after the operation on the 8th day in patient Sh, these PCs are detected, which may indicate the discovery of BBTB (Figures 6&7). Therefore, in patients with the same diagnosis, the permeability of BBTB is different both in the preoperative period and after. Therefore, when conducting chemotherapeutic treatment in patients, it is necessary to conduct individual monitoring of BBTB permeability in case of malignant brain gliomas. In patient T. with spinal hernia (Figures 8&9), PCs above 150nm were not detected before and after the operation, which confirms the assumption about the role of BBTB permeability in the appearance of PC of tumor origin in blood plasma. Most studies are devoted to establishing the relationships between protein groups of albumin and globulin fractions by radiation intensity. In addition, there was an increase in the concentration of albumin (1-10nm) in the blood compared with globulins (11-30nm) in the presence of oncology and other types of diseases [29]. In our studies, for the first time in malignant gliomas of the brain, a significant role was observed in the appearance of large protein complexes (above 150nm) in the blood plasma, which clearly indicate their tumor origin.
Figure 2: The distribution chart of the hydrodynamic diameter of the patient’s PC of A. (glioblastoma) in the measured blood plasma volumes before surgery.

Figure 3: The distribution chart of the hydrodynamic diameter of the patient’s PC of A. (glioblastoma) in the measured blood plasma volumes on the 8th day after the operation.
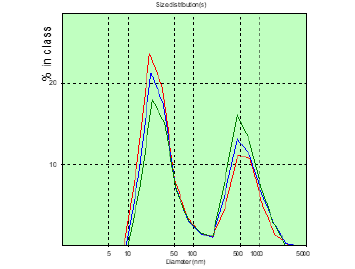
Figure 4: The distribution chart of the hydrodynamic diameter of the patient’s PC of M. (glioblastoma) in the measured blood plasma volumes before surgery.
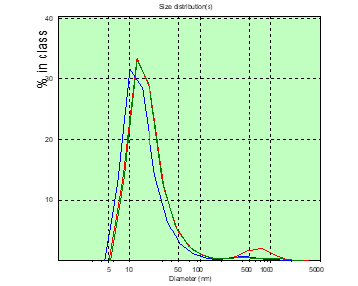
Figure 5: The distribution chart of the hydrodynamic diameter of the patient’s PC of M. (glioblastoma) in the measured blood plasma volumes on the 8th day after the operation.
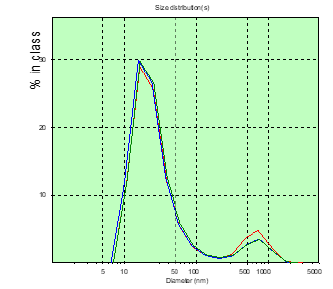
Figure 6: The distribution chart of the hydrodynamic diameter of the patient’s PC of Sh. (glioblastoma) in the measured blood plasma volumes before surgery.
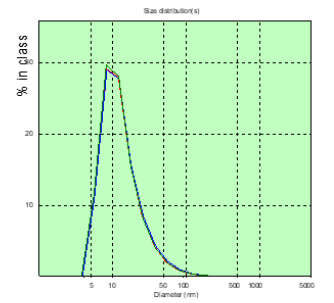
Figure 7: The distribution chart of the hydrodynamic diameter of the patient’s PC of Sh. (glioblastoma) in the measured blood plasma volumes on the 8th day after the operation.
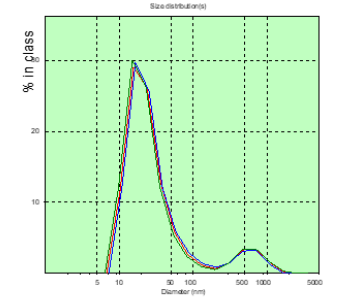
Figure 8: The distribution chart of the hydrodynamic diameter of the patient’s PC of T. (spinal gernia) in the measured blood plasma volumes before surgery.
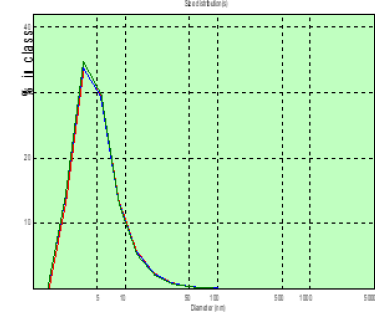
Figure 9: The distribution chart of the hydrodynamic diameter of the patient’s PC of T. (spinal gernia) in the measured blood plasma volumes on the 8th day after the operation.
Conclusion
Malignant CNS gliomas are characterized by infiltrative growth in the tissue surrounding the tumor, where the tumor cells migrate, which are almost impossible to remove simultaneously with the bulk of the tumor node. A study of the characteristics of tumor-associated inflammation in brain gliomas showed that tumor-infiltrating cells can release a wide variety of growth factors, cytokines/chemokines, and enzymes that support tumor growth, angiogenesis, and invasion. To destroy the cells of malignant gliomas in the postoperative period, a chemotherapeutic effect is used. However, malignant brain tumors are located in the barrier space, as a result of which many antitumor drugs can hardly penetrate or do not penetrate the blood-brain barrier (BBB) at all. The microvessels of tumor tissues also possess BBB properties and form a blood-brain/tumor barrier (BBTB), which helps to reduce the penetration of most chemotherapy drugs to the tumor site [21]. In fact, the functioning of the barriers prevents the creation of therapeutic concentrations of chemotherapy drugs in the area of infiltration of malignant cells. One of the methods for determining the degree of destruction of the BBB is an enzyme-linked immunosorbent assay, which determines the increase in blood activity of neurospecific enolase (NSE), and the amount of gliofibrillar acid protein (GFAP). Determination of their concentration in the blood plasma of patients with neuropathology makes it possible to assess the degree of BBB permeability and analyze damage to the neuronal and glial components of brain tissue, respectively [30-34]. It is known that malignant gliomas secrete protein complexes that can destroy the selective permeability of BBTB, which contributes to their appearance in the peripheral bloodstream. However, enzyme-linked immunosorbent assay is impractical to use to determine proteins of tumor origin in the blood plasma of a patient with brain tumors. Protein structures will penetrate through the BBTB into the blood plasma in a greater dependence on their volumes, compared with their molecular weight. The basis for the development of the BBTB permeability method is the detection of tumor originating blood plasma in the blood plasma of patients with malignant gliomas using the dynamic light scattering method, which allows analyzing the hydrodynamic diameter and the ratio of volume of blood cells appearing in blood plasma.
The detection of PC with a diameter of more than 150nm in the blood plasma of patients with malignant gliomas indicates an increased permeability of BBTB and BBB. The results of these studies are taken as the basis for the development of the express method for determining the increased permeability of BBTB in neurosurgical patients. The method can be used at a certain stage in the treatment of patients with malignant gliomas of the brain, using chemotherapeutic drugs depending on the permeability of the BBTBB and the BBB.
References
- Barnett CE (1946) Some applications of wave-length turbidimetry in the infrared. J Phys Chem 46(1): 69-75.
- Yu Z, Reid J, Yang Y (2013) Utilizing dynamic light scattering as a process analytical technology for protein folding and aggregation monitoring in vaccine manufacturing. J Pharm Sci 102(12): 4284-4290.
- Saluja A, Fesinmeyer R, Hogan S, Brems DN, Gokarn Y (2010) Difusion and sedimentation interaction parameters for measuring the second virial coefficient and their utility as predictors of protein aggregation. Biophysical Journal 99(8): 2657-2665.
- Tiagarajan G, Semple A, James J, Cheung J, Shameem M (2016) A comparison of biophysical characterization techniques in predicting monoclonal antibody stability. MABS 8(6): 1088-1097.
- Lorber B, Fisher F, Bailly M, Roy H, Kern D (2012) Protein analysis by dynamic light scattering: Methods and techniques for students. Biochemisty and Molecular Biology Education 40(6): 372-382.
- Malm AV, Corbett CW (2019) Improved Dynamic Light Scattering using an adaptive and statistically driven time resolved treatment of correlation data. Scientific Reports 9: 13519.
- Naveen Jose, Gajanan P Deshmukh, Menon Rekha Ravindra (2019) Dynamic Light Scattering: Advantages and Applications. Acta Scientific Nutritional Health 3. (3): 50-52.
- Jörg S, McKenna SA, Patel TR (2016) Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev (2016) 8(4): 409-427.
- Ivanov YV, Van der Pol E (2010) Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost 8(12): 2596-2607.
- Petrova GP (2009) Optical properties of solutions consisting of albumin and γ-globulin molecules in different ratio modeling blood serum. Laser Phys 196: 1303-1307.
- Papok IM (2012) Using the dynamic light-scattering method for the analysis of a blood-serum model solution. Moscow Univ Phys Bull 67(5): 452-456.
- Kovalchuk Yu P (2005) Express diagnostics of the severity of urgent states according to homeostasis by laser correlation spectroscopy. Clinical and Laboratory Consultation 7: 21-23.
- Zdraevskaya ON (2006) Diagnostic significance of the method of laser correlation spectroscopy in inflammatory and tumor lung diseases. Clinical laboratory diagnostics 5: 21-24, 33.
- Petrova GP et al. (2002) Rayleigh scattering method in the diagnosis of cancer. On Sat scientific tr. Medical physics 156-167.
- Petrusevich YM, Petrova GP (1996) The method of light scattering measurement in tumor diagnostics. Proc SPIE/ed. Ivanov AV, Kazaryan MA 2728: 2-9.
- Alekseev SG et al. (2005) Multiparametrical Testing of Blood Proteins Solutions with Diagnostic Purpose. Proc SPIE / ed. Ivanov AV, Kazaryan MA 5973: 597301-597301-10.
- Gabrusiewicz K, Ellert-MF, Sielska M, Kaminska B (2009) Modulation of IL-10 and GM-CSF Production in Gliomas Leads to Decrease Tumor Growth/5th International Conference on Tumor Microenvironment: Progression, Therapy & Prevention. Versailles France October 20-24.
- Shu CW, Shao HL, Chi Shing C (2009) The Spatial Distribution of Various Subtypes of Tumor Associated Macrophages within a Murine Brain Tumor. 5th International Conference on Tumor Microenvironment: Progression, Therapy & Prevention. Versailles France October 20-24.
- Gridina NY (2013) Utilizing SPR as a novel technique to measure cell aggregation for ketamine treated brain gliomas. Cancer Oncol Res 1(1): 1-5.
- Gridina N, Dorozinsky G, Khristosenko R, Maslov V, Samoylov A et al. Surface Plasmon Resonance Biosensor. Sensors & Transducers 149(2): 60-68.
- Groothuis DR (2000) The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol 2: 45-59.
- Lebedev AD et al. (1987) Laser correlation spectroscopy in biology. K Science Dumka pp. 256.
- Bazhora Yu I, Noskin LA et al. (2002) Laser correlation spectroscopy in medicine. Odessa Druk pp. 400.
- Merkus HG (2000) Particle Size Measurements. Fundamentals, Practice, Quality. Springer pp. 533.
- Xu R (2002) Particle Characterization. Light Scattering Methods. Kluwer pp. 410.
- Arefyev IM, Eskov AN, Yudin IK (1979) Laser correlation spectroscope for immunological and virological analyzes. Honey Equipment 2: 30-34.
- Gridina NY, Gupal AM, Draguntsova NG, Tarasov AL, Chunikhin AYu (2016) Development of a method for program analysis of the redistribution of parameters of protein structures in patients with brain gliomas. Computer Mathematics 1: 119-124.
- Pluzhnikov MS, Govorun MI, Migmanova KL (2007) Possibilities of laser correlation spectroscopy of blood serum in the diagnosis of head and neck tumors. Russian Otorhinolaryngology Medical Scientific and Practical Journal 77.
- Babaeva AG, Gevorkyan NM, Zotikov EA (2009) The role of lymphocytes in the operational change of the tissue development program. M: Publishing House of RAMS.
- Ternovoi KS, Kryzhanovsky GN, Musiychuk YuI (1998) Classification of blood plasma research results using laser correlation spectroscopy based on semiotics of preclinical and clinical conditions. Ukr Biochem Journal 70(2) 53-65.
- Burgman GP (1978) Liquorological and some metabolic disorders in closed traumatic brain injury. Guide to Neurotraumatology ed. AI Arutyunova M 4(1): 142-147.
- Voskresenskaya O.N., Tereshchenko S.V., Sholomov I.I. Objective characteristics of the acute period of concussion. Neurosurgery. 2003. No. 4. P. 22 - 27. (Russian).
- Lebedev VV, Evdokimova NV (2007) The significance of certain factors in the development of intracranial purulent complications in neurosurgical patients. Neurosurgery 1: 8-13.
- Mozerov SA, Chairkin IN (2004) Expression of glial fibrillar protein in brain tumors of astrocytic origin and in peritumor tissue. Morphological statements 1-2.
© 2020 Gridina NYa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






