- Submissions

Full Text
Open Access Biostatistics & Bioinformatic
Quinacrine and Berberine as Antiviral Agents against Dengue and Zika Fever: In Silico Approach
Vivek Srivastava*
Department of Biotechnology, Rama University, India
*Corresponding author: Vivek Srivastava, Department of Biotechnology, Faculty of Engineering & Technology, Rama University Uttar Pradesh Kanpur, India
Submission: June 15, 2018;Published: July 25, 2018

ISSN: 2578-0247
Volume2 Issue2
Abstract
TDengue and Zika fever are mosquito-borne viral diseases that have rapidly spread in all over world. Currently there are no specific drugs for DENV and ZIKV infection. The recent outbreak of these viruses realized that there are major health risks, demands an enhanced surveillance and a need to develop novel drugs against them. Non-structural proteins NS5 and NS3 are essential for the replication of the flavi-viral RNA genome. Therefore its inhibition could be considered as a useful strategy for treatment of DENV and ZIKV infection. Quinacrine and Berberine had been docked with NS5-methyltransferase of Dengue virus and NS3 protein of ZIKV using Auto dock 4.2 tools. Quinacrine and Berberine showed binding affinity -6.83kcal/mol and -6.22kcal/mol with NS5-methyltransferase and -7.32kcal/mol and -8.03kcal/mol with NS3 protease of ZIKV, respectively. Observations discussion in review will be useful in designing single drug against both virus infections..
Keywords: NS3 protease; NS5-methyltransferase; Antiviral drugs; Dengue virus; Zika virus
Introduction
Dengue is the most prevalent mosquito-borne virus, with nearly 400 million annual cases worldwide [1]. Dengue fever and dengue hemorrhagic fever are the quickly spreading mosquito-borne diseases in the universe today, for an watched 30-fold expand from claiming accounted cases in the most recent 50 years a long time [2]. The WHO estimates that, globally, 2.5 billion people are at risk, and annually, 50-100 million people become infected, of which approximately 0.5 million require hospitalization [3] and 22,000 deaths occur each year in areas where it is endemic [4].
The dengue disease has four viral serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), and its spectrum ranges from asymptomatic infection to dengue fever, Dengue Hemorrhagic Fever (DHF), and Dengue Shock Syndrome (DSS), and may lead the patient to death [5]. All four serotypes of dengue virus are transmitted to humans by the Aedes aegypti and Aedes albopictus mosquitoes [6]. The positive-sense flavi-virus RNA genome of 11kb forms a single open reading frame that is translated into an ~370kDa poly protein precursor containing the structural proteins and seven non-structural proteins known as NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5. NS5 is about 900 amino acids long, largest (104kDa) and the most conserved protein in DENV. It is also a bi-functional enzyme with a methyl transferees domain (M Tase; residues 1-296) at its N-terminal end and a RNA-dependent RNA polymerase (RdRp; residues 320-900) [7] C-terminal end [8]. Its enzymatic activities form attractive target for antiviral development [9-11]. In this article, we comprises molecular docking of four antiviral drugs such as Quinacrine, Amodiaquine, Berberine and Pro-chlorperazine against NS5-methyltransferase of dengue virus using in silico approach.
While zika virus is a mosquito-borne flavi-virus that was initially recognized in Ugand, Africa [12] in 1947 in monkeys through a method that observed yellow fever. It was later distinguished in people in 1952 in Uganda and the United Republic of Tanzania. Congenital ZIKV syndrome includes microcephaly, spasticity, craniofacial disproportion, irritability, seizures and other brainstem dysfunctions [13] has caused a public health emergency of international concern [14]. Brazil without immunity in the population saw large numbers infected immediately as the virus was amplified in the population, resulting in thousands of pregnant women infected at once. The Brazilian ZIKV strain has been shown to cause birth defects in experimental models by targeting cortical progenitor cells, inducing cell death and impairing neurodevelopment [15]. Initially the Brazilian Ministry of Health advised reporting diagnosed cases of Zika as dengue, since the symptoms were in most of the cases similar to a mild case of the latter.
The first baby in the USA born with ZIKV occurred on January 16th, 2016 [16]. Amodiaquine [17], prochlorperazine [18], quinacrine [19], and Berberine [19] are promising drugs approved by Food and Drug Administration against dengue virus which also belong to Flaviviridae family [20]. The WHO Director-General declared on February 1st, 2016 that the cluster of microcephaly cas-es and other neurological disorders reported in Brazil constitutes a Public Health Emergency of International Concern [21], it has therefore been identified as a problem for the entire world to deal with NS3 protein of ZIKV [22]. Due to the absence of any relevant treatment, this is especially important for the rapid discovery of a drug against ZIKV. Special priority should be given to the anti virals that were active against other flavi viruses such as dengue virus, yellow fever, Japanese encephalitis, etc., and to a lesser degree, against other members of the flaviviridae family like Hepatitis C. There are many things that dengue and zika have in common like they both are mosquito borne viruses spread especially by the Aedes mosquito.
ZIKV consist of a single stranded, positive sense, 5’-capped RNA with genome size of around 11kb which immediately released into the cytoplasm following by cell entry [22]. There are 59 and 39 un-translated regions along with only one open reading frame which codes a poly protein that further cleaved into three structural proteins and seven non-structural proteins i.e., NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 [23]. Among them the NS3 and NS5 proteins play a central role, together they harbour most of the catalytic activities needed for capping and replication [22]. Here, we have discussed for four FDA approved drugs as Quinacrine, Amodiaquine, Berberine and Prochlorperazine against DENV as ligands and performed molecular docking analysis against NS3 protein of ZIKV in order to observe the binding affinity of these drugs. Among these four drugs, the drug showing minimum binding energy has been considered as the lead drug for further analysis [24].
According our in silico analysis Quinacrine, Amodiaquine, Berberine and Prochlorperazine have been docked with NS5-methyltransferase of Dengue viruses using Auto dock 4.2 tool. Quinacrine showed minimum binding energy -6.83kcal/mol with NS5-methyltransferase (PDB ID-3EVG), and Amodiaquine, Berberine and Prochlorperazine have -6.64kcal/mol, -6.22kcal/mol and 6.67kcal/ mol, respectively [25]. Further the best-docked complexes were analyzed through Python Molecular Viewer software for their interaction studies. Thus from the Complex scoring and binding ability its deciphered that Quinacrine is a good promising drug for NS5-methyltransferase of dengue virus as drug target. Observations may extend an assuring platform for developing anti-viral competitive inhibitors against DENV infection.
Zika virus (ZIKV) is a mosquito borne pathogen currently causing large epidemics in Brazil. Its infection can cause microcephaly, a serious birth defect during pregnancy. The recent outbreak of ZIKV in February 2016 in Brazil realized it as a major health risk, demands an enhanced surveillance and a need to develop novel drugs against ZIKV. In this article, we discussed molecular docking analysis of four known antiviral drugs such as Quinacrine, Amodiaquine, Berberine and Prochlorperazine against non structural 3 (NS3) protein of ZIKV using Auto dock 4.2 tools. The protease activity of NS3 is necessary for viral replication and its prohibition could be considered as a strategy for treatment of ZIKV infection. Amongst these four drugs, Berberine has shown lowest binding affinity of -8.03kcal/mol with NS3 protein. Other drugs Quinacrine, Amodiaquine and Prochlorperazine have -7.32kcal/mol, -7.31 and -7.17kcal/mol with NS3 protein, respectively. Further the docked complexes were analyzed through Python Molecular Viewer software for their interaction studies. Thus from the Complex scoring and binding ability its deciphered that Berberine is more potent drug for NS3 protein of ZIKV as drug target (Singh et al. 2017). Observations made in this study will be useful in designing noble potent inhibitors against ZIKV infection.
Discussion
Infection with one of four dengue virus serotypes (DENV1-4) can lead to febrile illness and flu-like symptoms, or can progress to the more severe dengue hemorrhagic fever or dengue shock syndrome. Development of the more severe hemorrhagic fever is more likely if recovery from infection by one dengue serotype is followed by subsequent infection by a second serotype. Individual MTase and RdRp domains in full-length NS5 have conserved protein folds. The MTase (residues 1-262) adopts the SAM-dependent methyl transferase fold composed of four helices surrounding a central 7-stranded β-sheet [8,26] is shown in Figure 1 and Table 1. The active site, containing a catalytic K61-D146-K180-E216 (KDKE) motif, is positioned in the center of the β-sheet.
Figure 1: Overall fold of the DENV NS5 monomer [26].
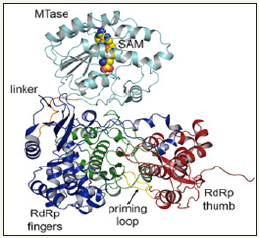
The docking result of these drugs with NS5-methyltransferase was shown in Table 1 which revealed that Quinacrine be a novel inhibitor for NS5-protein. Zika virus infection in humans is usually mild or asymptomatic. However, some babies born to women infected with Zika virus have severe neurological sequelae. An unusual cluster of cases of congenital microcephaly and other neurological disorders in the WHO Region of the Americas, led to the declaration of a public health emergency of international concern by the World Health Organization. In docking studies of known antiviral drugs with NS3-protein, best auto dock score was used as criteria to interpret the best conformation among the 10 conformations, generated by Auto Dock 4.2 program. The docking result of these drugs with NS3-protein was shown in Table 2 which revealed that Berberine be a novel inhibitor for NS3-protein.
Table 1: Docking results of known antiviral drugs with NS5-methyltransferase of dengue virus.

BE: Binding Energy; IME: Intermolecular Energy; IE: Internal Energy; TorE: Torsional; Energy; VdwE: vdW + Hbond + desolv Energy; EE: Electrostatic energy.
Table 2: Docking results of known ant0069viral drugs with NS3-Protein of Zika Virus.

BE: Binding Energy; IME: Intermolecular Energy; IE: Internal Energy; TorE: Torsional; Energy; VdwE: vdW + Hbond + desolv Energy; EE: Electrostatic energy.
Conclusion
The results obtained are useful in understanding the inhibitory mode of Quinacrine, Amodiaquine, Berberine and Prochlorperazine with catalytic site of NS5-methyltransferase and NS3-protein of dengue and zika virus respectively accurately predicting the activities of drugs on the basis of docking score of both. Here, we concluded that Quinacrine and Berberine are novel inhibitor for NS5-ptotein and of dengue and zika virus to prevent the dengue fever and zika fever respectively.
Acknowledgment
The author would like to thank Department of Biotechnology, Faculty of Engineering & Technology, Rama University Uttar Pradesh, Kanpur for providing facilities to carry out this review article.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504-507.
- World Health Organization (2009) World Health Organization and the Special Programme for Research and Training in Tropical Diseases (TDR). WHO, Geneva, Switzerland.
- http://www.who.int/mediacentre/factsheets/fs117/en/index.html
- http://www.cdc.gov/dengue/epidemiology/index.html
- Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11(3): 480-496.
- Wright PF, Durbin AP, Whitehead SS, Ikizler MR, Henderson S, Blaney JE, et al. (2009) Phase 1 trial of the dengue virus type 4 vaccine candidate rDEN4Δ30-4995 in healthy adult volunteers. Am J Trop MedHyg81(5): 834-841.
- Wyles DL (2013) Antiviral resistance and the future landscape of hepatitis C virus infection therapy. J Infect Dis 207(Supl 1): S33-S39.
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B (2002) An RNA cap (nucleoside-2’-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J 21(11): 2757-2768.
- Davidson AD (2009)Chapter 2: New insights into flavivirus nonstructural protein. Virus Res 74: 41-101.
- Noble CG, Shi PY (2012) Structural biology of dengue virus enzymes: towards rational design of therapeutics. Antiviral Res 96(2): 115-126.
- Lim SP, Noble CG, Shi PY (2015) The dengue virus NS5 protein as a target for drug discovery. Antiviral Res 119: 57-67
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, et al. (2014) Molecular evolution of Zika virus during its emergence in the 20th century. PLoSNegl Trop Dis 8(1): e2636.
- Costello A, Dua T, Dura P, Gülmezoglu M, Oladapo OT, Oladapo OT, et al. (2016) Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ 94(6): 406-406A.
- http://who.int/mediacentre/news/statements/2016/emergency-committee- zika-icrocephaly/en/2016
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, et al. (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534: 267-271.
- McNeil DGJ (2016) Hawaii baby with brain damage is first U.S. case tied to zika virus. The New York Times, New York, USA.
- Boonyasuppayakorn S, Reichert ED, Manzano M, Manzano M, Nagarajan K, et al. (2014) Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral Res 106: 125-134.
- Simanjuntak Y, Liang JJ, Lee YL, Lin YL (2015) Repurposing of prochlorperazine for use against dengue virus infection. J Infect Dis 211(3): 394- 404.
- Shum D, Smith JL, Hirsch AJ,Bhinder B, Radu C, et al. (2010) High-content assay to identify inhibitors of dengue virus infection. Assay Drug Dev Technol 8(5): 553-570.
- Tian H, Ji X, Yang X, Xie W, Yang K, et al. (2016) The crystal structure of Zika virus helicase: basis for antiviral drug design. Protein Cell 7: 450- 454.
- Anon (2016) WHO director-general summarizes the outcome of the emergency committee regarding clusters of microcephaly and guillain- barrésyndrome. WHO, Geneva, Switzerland.
- Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, et al. (2010) Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res 87(2): 125-148.
- Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc Goffart I, et al. (2014) Complete coding sequence of Zika virus from a French Polynesia outbreak in 2013. Genome Announc 2(3): e00500-e00514.
- Singh AP, Kumar A, Srivastava V (2017) In silico molecular docking of antiviral drugs against NS-3 protein of zika virus. International Journal of Scientific and Innovative Research 5(1): 37-40.
- Chaudhary S, Kumar A, Srivastava V (2017) In silico molecular docking of antiviral drugs against NS5-methyltransferase of dengue virus. International Journal of Scientific and Innovative Research 5(1): 41-44.
- Klema VJ, Ye M, Hindupur A, Teramoto T, Gottipati K, et al. (2016) Dengue virus nonstructural protein 5 (NS5) assembles into a dimer with a unique methyltransferase and polymerase interface. PLoSPathog 12(2): e1005451.
© 2018 Vivek Srivastava. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






