- Submissions

Full Text
Open Access Biostatistics & Bioinformatics
Formulation & Evaluation of Sustained Release Matrix Tablet of Repaglinide
Gaurav Agarwal*, Shilpi Agarwal and Shagun Goyal
RP Educational Trust Group of Institutions, India
*Corresponding author: Gaurav Agarwal, Faculty of Pharmacy, RP Educational Trust Group of Institutions, Karnal (Haryana), 132001, India
Submission: December 12, 2017; Published: February 23, 2018

ISSN: 2578-0247Volume1 Issue2
Abstract
The aim of present is to develop & evaluate Sustained release matrix tablet of Repaglinide. Repaglinide is an effective antihyperglycemic. But owing to its shorter half life it needs frequent administration. In present study, an attempt has been made to develop Sustained release matrix tablet of Repaglinide thereby reducing its frequency of administration & other dose related side effects. Various grades of HPMC (K4M, 100M & 15M) were used as hydrophilic matrix polymer. Croscarmellose was used as swelling agent. Total 9 formulations were prepared in trial batches. The formulation was evaluated for various pre compression & post compression parameters. All the formulations showed compliance with the pharmacopoeial standards. On the basis of evaluated parameters formulation F9 was considered to be the best one. Formulation F9 containing polymer HPMC showed 99.15% in- vitro drug release profile. The release data for formulation F9 was fitted to various mathematical models like zero order, first order, Krosmeyer Peppas & Higuchi model. It was observed that drug follows zero order & Higuchi model.
Keywords: Repaglinide; Sustained Release Matrix; HPMC (K4M, K100M, K15M); Croscarmellose; Wet Granulation
Introduction
Among the innumerable methods used for sustained release formulation matrix is the most popular approach. Hydrophilic or hydrophobic polymers are used as release retardants. Hydrophilic polymers are most widely used in matrix system because these are cost effective, make it easy to achieve desirable drug profile & have broader FDA acceptance. Drug release through matrix system is governed by water penetration, polymer swelling, drug dissolution & drug diffusion [1-3]. Diabetes Mellitus is a metabolic disorder resulting from defective insulin secretion, action or both [4]. Repaglinide is an effective antihyperglycemic used for the management of Type 2 Diabetes Mellitus. It is a benzoic acid derivative belonging to meglitinide class of oral hypoglycemic. After oral administration it is rapidly & completely absorbed & peak plasma concentration is achieved in approximately 1hr. It is removed from the blood stream within a span of 1hr. the mean absolute bioavailability is 56% when given with food [5]. Due to its shorter duration of action & faster onset of action it serves as good candidate for SR matrix tablets [5,6]. Numerous studies have been reported in the literature regarding the use of Hydroxy Propyl Methyl Cellulose (HPMC) to control the release of drug from matrices. Thus, in present study an attempt has been made to formulate sustained release matrix tablet of repaglinide & to evaluate the same for various parameters; in order to produce additional antidiabetic activity thereby resulting in dose reduction & its dose related side effects.
Materials and Method
Repaglinide was obtained as free gift sample from Torrent Pharmaceuticals Limited, Gujarat, India. HPMC K 100M, HPMC K4M, HPMC K 15M were purchased from yarrow chemicals products, Mumbai. Talc, PVP K30, Calcium Stearate were purchased from SD Fine chemicals Ltd., Mumbai. Croscarmellose sodium was offered as gift sample from Shreeji pharma International.
Formulation of Sustained Release Matrix Tablets
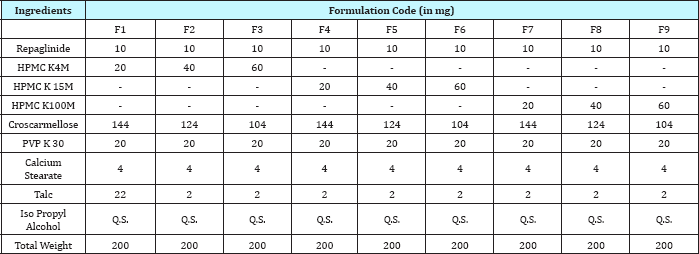
Total 9 formulations were prepared in trial batch by wet granulation method using Iso Propyl alcohol as non aqueous solvent. Proportion of different excipients with drug was as given in Table 1 were weighed individually. All ingredients were sifted through sieve no. 60. Half of the required quantity of all ingredients excluding calcium stearate and talc were mixed together for about 15 minutes. PVP K30 dissolved in isopropyl alcohol was used for the purpose of wet granulation of final blend. The damp wet mass was prepared in mortar and passed through sieve no. 12. The granules were dried in hot air oven at 60 °C for 30 minutes. The dried granules were then passed through sieve no. 16. Finally rest half quantity of the ingredients were added and mixed in the granules. The blend was then lubricated with calcium stearate and talc. Finally tablets were compressed to net weight of 200mg by using 10 station mini rotary tablet punching machine.
Table 1: Composition of matrix tablet of repaglinide.
Evaluation of Granules [7-10]
Angle of repose
To determine angle of repose the granules are allowed to flow freely through funnel on a plain surface. The diameter and height of the heap of the powder is determined & angle of repose of granules was determined by following equation
tanθ=h/r
Where h and r are height and radius of the powder cone respectively.
Bulk density
Loose bulk density & Tapped bulk density were determined using the following formulas.
Loose Bulk Density=Weight of Granules/Bulk Volume of Granules
Tapped Bulk Density=Weight of Granules/Tapped Volume of Granules
Compressibility index
The compressibility index of the granules was determined by using the following formula.
Carr's Index=Tapped Density-Loose Bulk Density/Tapped Density*100
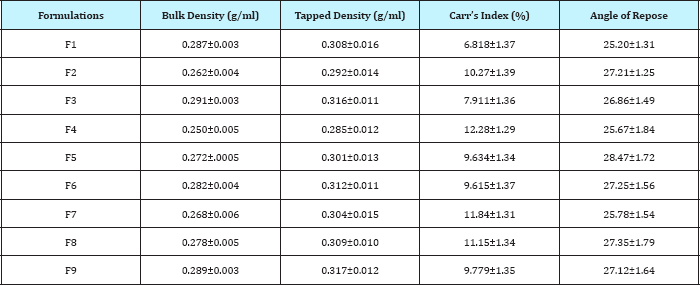
The evaluated pre compression parameters of granules are as shown in Table 2
Table 2: Pre compression parameters of granules.
Drug-excipient compatibility studies
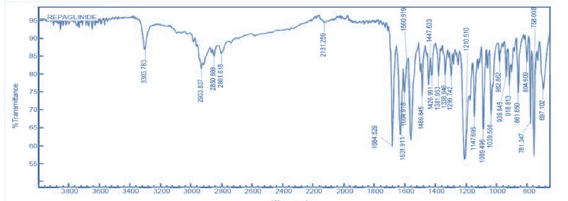
FTIR studies: The FT-IR spectrum of pure drug (Repaglinide) and its physical mixture with different grade of polymers and excipients is as shown in Figure 1 & 2 and illustrates the compatibility of drug with excipients.
Figure 1: FT-IR spectrum of pure repaglinide.

Figure 2: FT IR spectrum of repaglinide +HPMC K 100M.
Differential scanning calorimetry: A differential scanning calorimeter (DSC) measures the energy transferred to or from a sample undergoing a physical or chemical change. Differential scanning calorimeter indicates sharp endothermic peak at 132 °C for pure repaglinide. DSC thermogram of drug with different grades of HPMC (K4M, 15M & 100M) did not show any extra peak illustrating the compatibility of drug with excipient.
Evaluation of Tablets [7,9,10]
After compression tablets are evaluated for the following parameters.
Thickness & diameter
Physical dimensions of the tablet were evaluated by using vernier caliper. It was measured in mm.
Appearance & texture
Appearance, color, shapes & texture of the tablets was evaluated by visual inspection.
Hardness
Hardness of the tablets was evaluated by using Monsanto Hardness Tester & was expressed in kg/cm2
Friability
The test was carried out by using Roche fribilator. Pre weighed tablets were introduced into fribilator. The instrument was set at a speed of 25rpm & was operated for about 4 minutes. Tablets are weighed again & %age weight loss was determined by using following formula.
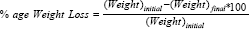
Weight variation
Twenty Tablets were weighed together and average weight was calculated. Then each tablet was weighed individually and mean weight was calculated. The % age difference between the two was calculated. The tablets pass the test if not more than 2 tablets fall outside the percentage limit and none of the tablet should deviate twice of the percentage limit.
Drug content uniformity
20 tablets were weighed and powdered. A quantity equivalent to 100mg of repaglinide was weighed and transferred to 250ml of volumetric flask. 150ml of 0.1N HCl was added; shaken well and sonicated for about 25-30 minutes. The final volume was made up to 250ml using 0.1N HCl. The solution was filtered and 10ml of filtrate was taken in volumetric flask. The final volume was made up to 100ml with 0.1N HCl. The absorbance of the resulting solution was measured at 242nm using UV Spectrophotometer. The concentration of the drug in the tablet powder was calculated by using the following equation:
Cu /Cs =Au /As *Dilution Factor
Where Cu=Concentration of unknown sample
Cs=Concentration of standard sample
Au=Absorbance of unknown sample
As=Absorbance of standard sample
In vitro Dissolution studies
In- vitro dissolution studies of the prepared tablets was conducted by using USP Type II Apparatus (Paddle Type) at 37±0.5 °C. The paddle was rotated at a speed of 50rpm. The dissolution studies of prepared tablets of repaglinide was carried out first in 0.1N HCl at pH 1.2 for about 2hrs, then these tablets are transferred in phosphate buffer (pH 7.4) and dissolution was carried out. 5ml of dissolution fluid was withdrawn at regular intervals and filtered through 0.45|im filter paper. Content of drug in each sample was determined by using UV spectrophotometer at 242nm for 0.1N HCl & at 278nm for phosphate buffer. The sample withdrawn was replenished with fresh sample after every withdrawal in the dissolution flask. On the basis of the dissolution studies the formulation that gives the best release profile of the drug was considered as the optimized formulation. The dissolution profiles of the different formulations are as shown in Figure 3-7.
Figure 3: DSC thermogram of repaglinide.
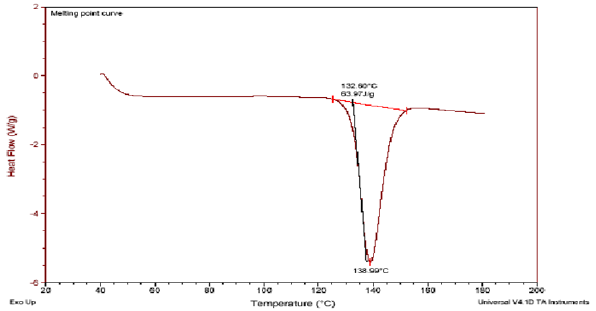
Figure 4: DSC thermogram of repaglinide with HPMC K (100M, 15M & 4M).
Figure 5: In-Vitro dissolution profile of formulation F1-F3.
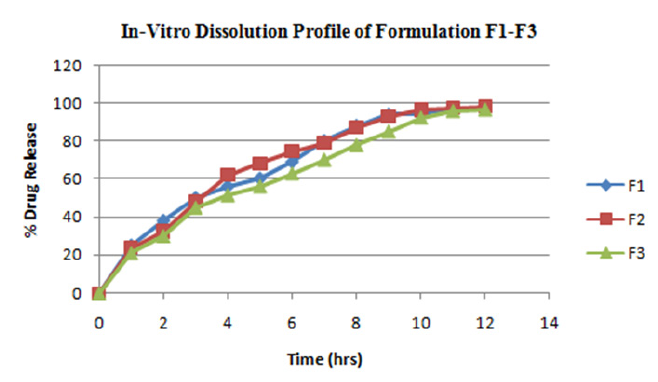
Figure 6: In-vitro dissolution profile of formulation F7-F9.
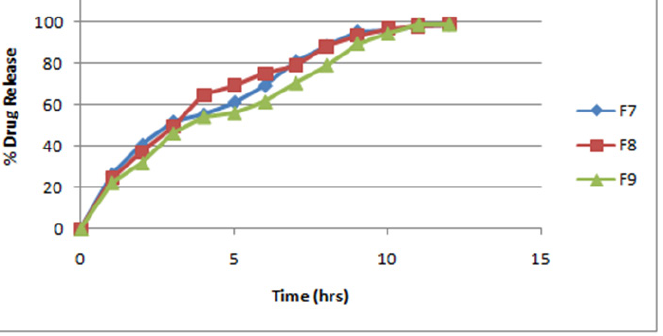
Figure 7: Graphical representation of formulation F9: zero order kinetics, first order kinetics, higuchi model & korsmeyer peppas model.
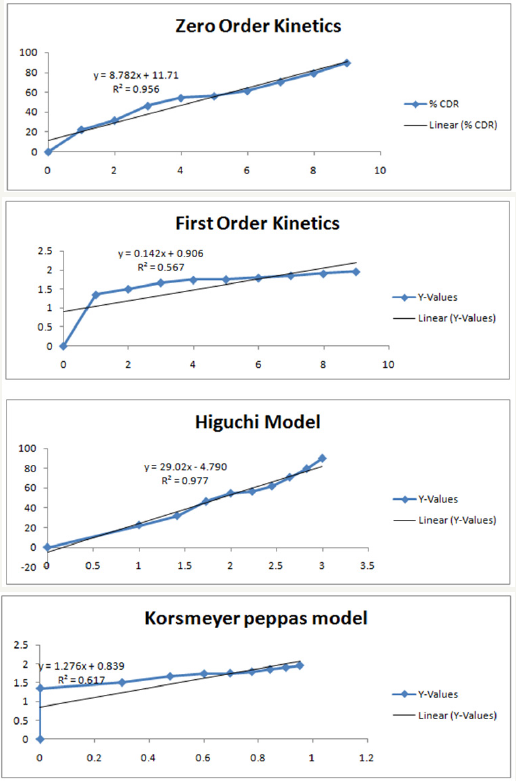
On the basis of studies it has been concluded that an increase in the concentration of the polymer retards the drug release from the matrices. To establish the order and mechanism of drug release, dissolution data of the optimized batches were fitted to four different kinetic models, namely, Zero order model, first order model, Higuchi model and Korsmeyer peppas model. The model for best fit was predicted from the value of R2. For an ideal fit, value of R2 was 1. Hence, the model which gives the R2 value nearest to 1 describes the order of drug release. From the results of data fitting to various models (Table 3-8), it was found that the optimized batch F9 showed zero order drug release & followed Higuchi model describing drug release from polymeric matrix.
Table 3: FTIR spectral data of pure repaglinide.
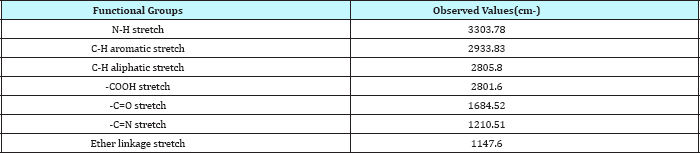
Table 4: FTIR spectral data of repaglinide+HPMC K1GGM.
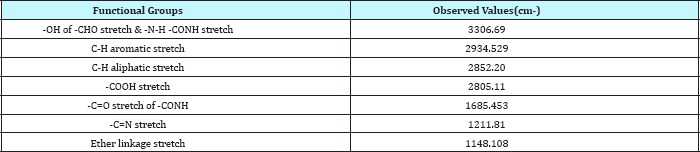
Table 5: Evaluation of repaglinide sustained release tablets.
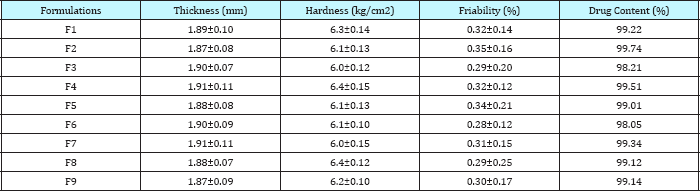
Table 6: In-vitro dissolution profile of formulations F1-F9.
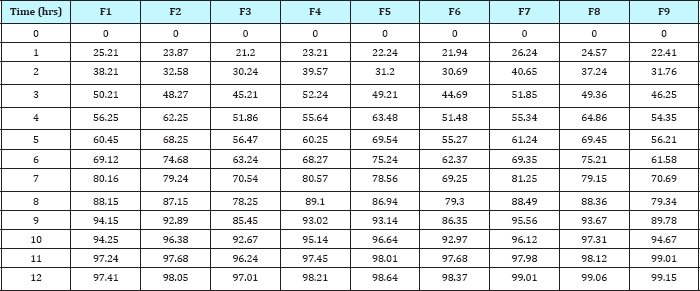
Table 7:In-vitro release data of formulation F9: zero order kinetics, first order kinetics, higuchi model and korsmeyer peppas model.
Table 8: Value of R2 obtained from different kinetic models.

Results & Discussion
The formulations F1 to F9 showed acceptable range of different pre-compression & post compression parameters like angle of repose, Carr's index, hardness, thickness, friability, drug content etc. FTIR spectrum & DSC thermogram illustrates the compatibility of drug with excipients. The matrix tablets of formulations F1 to F3 consist of HPMC K 4M as polymer. The release rate of the formulation was in order F1>F2>F3 up to 3hrs then F2> F1>F3 up to 10-11hrs. The release rate of formulation F3 was 97.01 at an interval of 12hrs & of formulation F2 was found to be 98.05 at 12hrs. The order of drug release for formulation F4 to F6 containing polymer HPMC K 15M was found to be F4>F5>F6 up to 3hrs, then F5>F4>F6 up to 11hrs. The release rate of formulation F6 was found to be 98.37 & of formulation F5 was found to be 98.64 at 12hrs. For formulations F7-F9 containing polymer HPMC K 100M the order of drug release was found to be F7>F8>F9 up to 3hrs. Then F8>F7>F9 up to 10hrs: F9>F8>F7 for 11-12hrs. The formulation F9 shows most significant result in terms of sustained release & in-vitro dissolution rate. Thus Formulations F7-F9 containing polymer HPMC K 100M were found to be the best one.
Conclusion
Repaglinide sustained release matrix tablets were successfully prepared by using various grades of HPMC as polymer to retard the release and achieve the retard dissolution profile. Drug & polymer were found to be compatible as indicated by FTIR studies & DSC thermogram. From the observations it was concluded that polymers used in different concentrations differ in their ability to sustain the drug release. Further it was concluded that polymer HPMC K100M showed better extended release property than HPMC K4M & HPMC K15M used in formulation of sustained release matrix tablet. It was found that drug release from the matrix tablets was decreased with increase in drug polymer ratio. Release rate of the drug from the matrix was significantly influenced by proportion of swelling of HPMC. It may be concluded from the present study that slow & controlled release of repaglinide over a period of 12hrs was obtained from formulation F9 using polymer HPMC K100M. The drug release kinetics revealed zero order release pattern & followed higuchi model. Formulation & evaluation of SR matrix tablet of repaglinide was found to be satisfactory. On the basis of various evaluated parameters formulation F9 was considered to be the best one. The results of release studies indicated the possibility of achieving a suitable modulation of repaglinide by varying the concentrations of polymers (HPMC K4M, K15M, and K100M) in the tablet & could lead the market with respect to their counterparts.
References
- Reddy DV, Rao AS (2014) Formulation and evaluation of extended release tablets of tapentadol hydrochloride using hydrophilic- hydrophobic polymer combinations. J Pharma Res 8(10): 1368-1374.
- Kumar V, Prajapati SK, Soni GC (2012) Sustained release matrix type drug delivery system: a review. World Journal of Pharmacy and Pharmaceutical Sciences 1(3): 934-960.
- Maderuelo C, Zarzuelo A, Lanao JM (2011) Critical factors in the release of drugs from sustained release hydrophilic matrices. Journal of Controlled Release 154(1): 2-19.
- American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes care 27(suppl 1): s5-s10.
- Ambavane V, Patil R, Ainapure SS (2002) Repaglinide: a short acting insulin secretagogue for postprandial hyperglycemia. Journal of postgraduate medicine 48(3): 246-248.
- Owens DR (1998) Repaglinide-prandial glucose regulator: a new class of oral antidiabetic drugs. Diabetic medicine 15(Suppl 4): S28-36.
- Arakeri V, Kumar PA, Kulkarni SV (2014) Design and in-vitro evaluation of sustained release matrix tablets of Repaglinide. International Journal for Pharmaceutical Research Scholars 3(4): 142-153
- Joshi J, Bhakuni L, Kumar S (2012) Formulation and evaluation of solid matrix tablets of repaglinide. Der Pharm Sin 3(5): 598-603.
- Barot N, Darshan M, Praful DB (2014) Formulation development and evaluation of sustained release matrix tablets of repaglinide. International Journal of Pharmaceutical Research and Bio-Science 3(2): 370-396.
- Sarfaraz MD, LingarReddy B, Rajagopal UH (2013) Design and in vitro evaluation of gastro retentive floating tablets of Repaglinide. International Journal of drug delivery & research 5(3): 322-332.
© 2018 Gaurav Agarwal, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






