- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Γ-H2ax Histone as a Biomarker of Medicinal Plants Genotoxicity: Technical Note
Mélanie Poivre1,2*, Marie-Hélène Antoine1, Amandine Nachtergael2, Pierre Duez2 and Joëlle Nortier1
1Laboratory of Experimental Nephrology, Faculty of Medicine, Université Libre de Bruxelles, Belgium
2Laboratory of Therapeutic Chemistry and Pharmacognosy, Faculty of Medicine, Pharmacy and Biomedical Sciences, Research Institute for Health Sciences and Technology, University of Mons-UMONS, Belgium
*Corresponding author:Poivre Mélanie, Laboratory of Experimental Nephrology, Faculty of Medicine, Université Libre de Bruxelles, Campus Erasme, 808 route de Lennik, 1070 Brussels, Belgium
Submission: January 31, 2024;Published: February 14, 2024

ISSN:2640-9208Volume7 Issue4
Abstract
Natural products are generally considered as safe and reliable, whereas they could represent a real danger for public health. Indeed, many herbs are either directly reactive towards DNA or likely to disturb cellular homoeostasis, cell cycle and/or genome maintenance mechanisms. Genotoxicity refers to the deleterious effect of a chemical compound or a physical event on the genetic material. Such genotoxic events are considered hallmarks of cancer risk. In this technical note, discussions are held about manipulations involving the use of γ-H2AX as biomarker of genotoxicity through assessment of two medicinal plants involved in aristolochic acid nephropathy patients in Belgium: Aristolochia species and bark of Magnolia officinalis. γ-H2AX is increasingly considered as an attractive biomarker for either DNA damage or DNA repair. Moreover, the detection of γ-H2AX can potentially serve as a biomarker for transformation of normal tissue to malignant tissues in many cancers.
Keywords: Aristolochia; Magnolia; Genotoxicity; Apoptosis; Γ-H2AX histone; Aristolochic acids
Introduction
According to the WHO, cancer is the second leading cause of death globally, accounting for an estimated 9,6 million of deaths or 1 in 6 deaths, in 2018. Remarkably, already in 2012, the WHO had estimated that 30% of cancer mortalities were due to lifestyle choices and environmental factors that could and should be avoided [1]. Emergence of DNA mutations, especially Double-Strand Breaks (DSB) are among the first procedures taking place in cancer formation and progression because of endogenic and exogenic factors [2]. Although often perceived as innocuous, many herbs contain phytochemicals that are either directly reactive towards DNA or likely to disturb cellular homeostasis cell cycle and/or genome maintenance mechanisms; this may translate into genotoxicity or carcinogenicity [1]. In this context, many organizations such as The Organization for Economic Co-Operation and Development (OECD), the Food and Drug Administration (FDA) the International Agency for Research on Cancer (IARC), the European Medicines Agency (EMA) and the European Centre for the Validation of Alternative Methods (ECVAM) investigated the validation of tests and provided a general framework, practical approaches and rules for data interpretation [3]. Guidelines have been established by OECD, ICH and EMA committees [3-5] to optimize genetic toxicology testing for the prediction of potential human risks [1].
Histone modifications play fundamental roles in most biological processes involved in the manipulation and expression of DNA [6]. H2AX constitutes 2 to 25 % of mammalian histones H2A depending on the organism and cell type [7]. H2A histone together with H2B, H3 and H4 makes an octamer of core histone proteins, around which 145-147 base pairs of DNA are wrapped [8]. This unit is called a nucleosome, which is the smallest subunit of genomic DNA in eukaryotic cells [8]. When a Double‐Strand Break (DSB) occurs in the DNA, many molecules of histone H2AX present in the chromatin flanking the break site are rapidly phosphorylated [9]. The phosphorylated derivative of H2AX is named γ‐H2AX and the phosphorylation site is a conserved serine four residues from the C‐terminus, 139 in mammals and 129 in budding yeast [9,10]. A γ‐H2AX antibody reveals that the molecules form a γ‐focus at the DSB site. The γ‐ focus rapidly increases in size for 10-30 min after formation and remains until the break is repaired [9]. These proteins assemble in nuclear domains, named “DNA damage foci” that can be visualized cytologically by immunofluorescence with antibodies specific to the phosphorylated form [11].
Therefore, γ-H2AX is increasingly considered as an attractive biomarker for either DNA damage or DNA repair [12,13]. Measuring γ-H2AX to monitor DNA damage and repair offers some key experimental advantages, being technically simpler, more precise and sensitive than other available techniques [9]. Moreover, the detection of γ-H2AX can potentially serve as a biomarker for emergence of malignant tissues, especially in breast, lung, colon, cervix and ovary cancers [13,2]. In this technical note, in vitro experimental protocols focusing on the use of γ-H2AX as biomarker of genotoxicity were held through assessment of two medicinal plants involved in aristolochic acid nephropathy patients in Belgium: Aristolochia species and bark of Magnolia officinalis. These patients ingested root extracts of these plants imported from China for slimming purposes and further developed severe to endstage kidney disease and for a dramatic proportion of them, upper urinary tract and bladder urothelial carcinoma [14].
Case Report
Herbal extraction
As previously described by Poivre et al. [15] and Nachtergael et al. [16] dried Magnolia officinalis Rehder & E.H. Wilson cortex was obtained from Phytax (Schlieren, Switzerland). Certificate of analysis was obtained from the company, indicating a sample free from aflatoxins (detection limit, 0.4μg/kg) and heavy metals (detection limit, 10μg/kg). Instead of the Aristolochic Fang Chi, YC Wu, ex LD Chow & SM Hwang, initially ingested and now prohibited in Belgium, Aristolochia baetica L. radix was used during manipulations. It is deposited at the National Herbarium of Morocco (Scientific Institute of Rabat) under the voucher name RAB 78463. Further investigations proved that A. baetica L. also contains aristolochic acids [16,17]. Each material was decocted in water, following traditional instructions in the Chinese Materia Medica [18]. The decoction was filtered on cellulose 3 times, the filtrate was lyophilized (Heto Power Dry LL1500, Thermos Fisher Scientific Inc, Waltham, USA) and stored at -20°C until further use. The extracts were dissolved and diluted with a complete culture medium to the required concentrations.
Cell culture
The FHs 74 Int human intestinal epithelial cell line (ATCC number CCL-241) was obtained from the American Type Culture Collection (St Cloud, USA). Cells were cultured in DMEM high glucose (4.5g/L) medium supplemented with 10% FBS Gold, 10 mM HEPES, 2mM L-glutamine, 1% non-essential amino acid, 100 000U/L penicillin, 100mg/L streptomycin, 30ng/μL EGF and 10μg/ μL insulin and maintained at 37 °C in a humidified atmosphere of 5% CO2 in air [16].
γ-H2AX detection by immunofluorescence
Cells were seeded in 6-well plates (3 x 104 cells per well) and grown at 37 °C for 22-26 h before dilution of plant extract (1mg/ mL) or positive control (bleomycin, 200μg/mL) or negative control (supplemented medium) was added. Bleomycin was chosen as a positive control because of its well-known genotoxicity and its capacity to generate double-strand breaks [18,19]. After 24 hours and 48 hours, the cells were washed with PBS, fixed with 1mL/well of ice-cold methanol for 10min, washed with PBS, treated for 1h at room temperature with 1mL/well of blocking solution (1% BSA in PBS) and washed again. The cells were incubated overnight at 4 °C with 250μL/well of a 1/1000 dilution of the anti- γ-H2AX primary antibody, washed with PBS, incubated 1h at room temperature with 500μL/well of a 1/1000 dilution of the Daylights 488 conjugated secondary antibody, washed with PBS and incubated for 30 min at 37 °C with 1 mL/well of a solution of propidium iodide (1μg/mL)/ RNase A (10μg/mL) in PBS. The fluorescence was quantified on 10 microscope fields in 3 biological replicates (20x magnification) using an epifluorescence microscope (Nikon Eclipse 80i, Nikon Instruments, Amsterdam, Netherlands) equipped with a DS-5M-L1 digital camera, driven by the NIS-Element Viewer software (Nikon Instruments). On each field, the total γ-H2AX fluorescence intensity was corrected by the cell number [16].
Results
In this study, Aristolochia and Magnolia aqueous extracts, alone or in combination, were evaluated for their genotoxicity using immunodetection of the phosphorylated histone γ-H2AX. Typical immunofluorescence images were obtained for controls and exposed FHs74 intestinal cells over 24h and 48h (20x magnification) repeated in 3 independent experiments (Figure 1). Focusing on the γ-H2AX’s appearance on FHs74 intestinal cell line, phosphorylated histone was detected in foci after 24 hours of exposure to the positive control and A. baetica-M. officinalis mixture [16]. These results suggest that herbal products mixture generate DNA damages, probably DSB damages (Figures 1 & 2). However, γ-H2AX’s appearance is not similar after 48 hours of exposure to bleomycin and A. baetica-M. officinalis mixture. In these conditions, γ-H2AX appears as a ring around nuclei. This is probably an apoptotic ring. Indeed, Solier et al. [20] have shown that γ-H2AX can both appear during apoptosis (than with a “apoptotic ring” form) or in presence of DNA damages (with a foci appearance) [20]. These results therefore might probably reveal the presence of apoptosis in FHs74 intestinal cells after 48 hours of treatment (Figure 2).
Figure 1:Typical immunofluorescence images obtained for control and exposed FHs74 intestinal cells over 24 h and 48 h (20x magnification; repeated in 3 independent experiments).
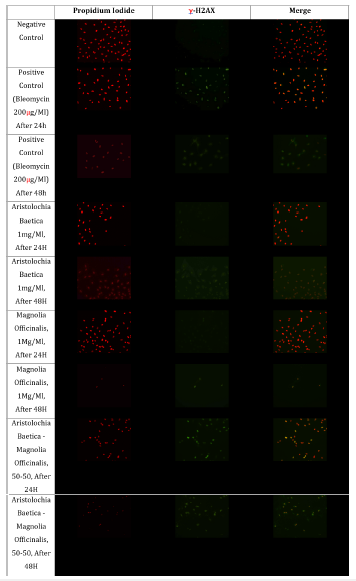
Figure 2:γ-H2AX’s appearance after 24h and 48h of FHs74 intestinal cells exposed to A. baetica-M. officinalis mixture (1mg/mL) Gain: 1.03; Exposure time: TRITC -80 msec and FITC-200 msec.

Discussion
Solier et al. [12] discovered the apoptotic ring, which microscopically looks like a nuclear annular staining early in apoptosis. This ring is, in three-dimensional space, a thick intranuclear shell consisting of epigenetic modifications including histone H2AX and DNA damage response (DDR) proteins [20]. During apoptosis, γ-H2AX is initiated in the nuclear periphery immediately inside the nuclear envelope while total H2AX remains distributed throughout the nucleus. This process is readily detectable by immunofluorescence microscopy and is known as the “γ-H2AX ring” [12,21]. After 48 hours of cell exposure, γ-H2AX’s appearance suggests that both positive control and herbal mixture (Aristolochia-Magnolia) result in apoptosis in FHs74 intestinal cell line. The present results, in addition to relevant data previously published by colleagues, confirm that γ-H2AX can be considered as an attractive biomarker for either DNA damage or apoptosis [14- 16]. The apoptotic γ-H2AX ring might revive general interest in the field of nuclear apoptosis. This apoptotic “ring pattern” with dual reactivity to γ-H2AX could be a useful tool for basic cellular biology research, but also to score apoptosis vs DNA damage response in clinical samples collected to monitor the efficacy of anticancer regimens [20,22]. γ-H2AX seems to be a reliable biomarker of genotoxicity of herbal products. This is of particular interest as genotoxicity seems to be linked to the development of certain cancers associated to the widely unregulated or over-the-counter use of herbal products.
Conclusion
Maintaining the integrity of the genome is essential for all living organisms. Indeed, un-, or mis-repaired DNA damage can lead to mutations, cell death and even cancer [13]. Screening for DNA damage represents a major public health challenge. In this context, histone γ-H2AX has been shown to be a sensitive marker of DNA damage, particularly double-strand breaks and even cell apoptosis [12,13]. In addition, people are more and more confident that herbal products are safer than allopathic medicines. More evaluations of these natural products are therefore needed. These technical note shows the appearance of γ-H2AX foci in FHs74 intestinal cells after 24 hours’ exposure to Magnolia officinalis and Aristolochia baetica aqueous extracts, and the appearance of an apoptotic ring after 48 hours’ exposure using immunofluorescence detection. However, these results require further investigations to confirm that apoptosis is involved.
References
- Poivre M, Nachtergael A, Bunel V, Okusa P, Duez P (2017) Chapter 9: Genotoxicity and carcinogenicity of herbal products. In Toxicology of Herbal Products, pp. 179-215.
- Palla VV, Karaolanis G, Katafigiotis I (2017) Gamma-h2ax: can it be established as a classical cancer prognostic factor? Tumor Biol 39(3): 1010428317695931.
- EMEA (2008) Guideline on the assessment of genotoxicity of herbal substances/preparations.
- OECD (2014) OECD guidelines for testing of chemicals.
- ICH (2015) Guideline on the need for carcinogenicity studies of pharmaceuticals S1A.
- Bannister A, Kouzarides T (2011) Regulation of chromatin by histone modifications Cell Res 21(3): 381-395.
- Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, et al. (2009) H2ax: Functional roles and potential applications. Chromosoma 118(6): 683-692.
- Ausio J (2006) Histone variants-the structure behind the function Brief Funct Genomic Proteomic 5(3): 228-243.
- Asako N, Olga A, Christophe R, Duane RP, Sinogeeva NI, et al. (2006) Techniques for γ‐h2ax detection. Methods in Enzymology 409: 236-250.
- Kuo LJ, Yang LX (2008) γ-H2AX - A novel biomarker for DNA double-strand breaks. In Vivo 22(3): 305-309.
- Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S (2013) H2AX as a marker of DNA double strand breaks and genomic instability in human population studies Mut Res 753(1): 24-40.
- Solier S, Pommier Y (2009) The apoptotic ring: A novel entity with phosphorylated histones H2AX and H2B and activated DNA damage response kinases. Cell Cycle 8(12): 1853-1859.
- Fan T, Kang H, Wu D, Zhu X, Huang L, et al. (2022) Arabidopsis γ-H2A. X-Interacting protein participates in DNA damage response and safeguards chromatin stability. Nat Commun 13(1): 7942.
- Bunel V, Souard F, Antoine MH, Stévigny C, Nortier J (2018) Nephrotoxicity of natural products: aristolochic acid and fungal toxins. Comprehensive Toxicology (Third Edition) 14: 340-379.
- Poivre M, Antoine MH, Kryshen K, Atsapkina A, Shikov et al. (2023) Assessment of the cytotoxicity, mutagenicity and genotoxicity of two traditional chinese herbs: Aristolochia baetica and magnolia officinalis. Toxins 15(1): 52.
- Nachtergael A, Poivre M, Belayew A, Duez P (2015) In vitro genotoxicity tests point to an unexpected and harmful effect of a Magnolia and Aristolochia association. J Ethnopharmacol 174: 178-186.
- Yamani A, Bunel V, Antoine MH, Husson C, Stévigny C, et al. (2015) Substitution between aristolochia and bryonia genus in north-eastern Morocco: Toxicological implications. J Ethnopharmacol 166: 250-60.
- Bensky D, Clavey S, Stõger E (2004) Materia medica-Chinese herbal medicine. In: (3rd edn), Eastland Press, Seattle, USA, pp. 3-6.
- Chen J, Ghorai MK, Kenney G, Stubbe J (2008) Mechanistic studies on bleomycin-mediated DNA damage: multiple binding modes can result in double-stranded DNA cleavage. Nucleic Acids Res 36(11): 3781-3790.
- Solier S, Pommier Y (2014) The nuclear γ-H2AX apoptotic ring: implications for cancers and autoimmune diseases. Cell Mol Life Sci 71(12): 2289-2297.
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM (2000) Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 275(13): 9390-9395.
- Solier S, Kohn KW, Scroggins B (2012) Heat shock protein 90α (HSP90α), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc Natl Acad Sci USA 109(32): 12866-12872.
© 2024 Mélanie Poivre. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






