- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Effect of Promax-C on Type 2 Diabetes and Oxidative Stress Induced by Hypercaloric Sucrose Diet Combined with Dexamethasone in Rats
Didier Beyssiri1*, Faustin Dongmo1, Daniel Assimilay1, Jidibe Pierre1, Kidjama Ngo Ngimout1, Imar Djibrine Soudy2, Tchuenguem Fohouo FN1 and Dongmo SS1
1Department of Biological Sciences, Faculty of Sciences, University of Ngaoundéré, Cameroon
2University Institute of Scienced and Technology (UIST) of Abéché, N’djamena, Tchad
*Corresponding author:Beyssiri Didier, Department of Biological Sciences, Faculty of Sciences, University of Ngaoundere, Cameroon
Submission: August 10, 2023;Published: October 18, 2023

ISSN:2640-9208Volume7 Issue3
Abstract
The antidiabetic and antioxidant effects of PROMAX-C were evaluated in male rats fed by hypercaloric sucrose diet supplemented with administration of dexamethasone. PROMAX-C at doses of 37.5, 75, 150 and 300mg/kg was administered by gavage in rats to assess its effect on the oral glucose tolerance test. The effects of this drug were also evaluated in a model of Type 2 Diabetes induced by a hypercaloric sucrose diet (MACAPOS 1 model) simultaneously with intraperitoneal administration of dexamethasone at a dose of 0.2mg/kg for 30 days. Metformin (20mg/kg) was administered as the reference drug. In the oral glucose tolerance test, administration of PROMAX-C showed a maximum reduction in blood glucose of 34.8% for the 300mg/kg dose 120 minutes after administration of PROMAX-C. This dose also significantly prevented increases in mean blood glucose (47.76%), triglycerides (41.38%), LDL cholesterol (61.88%), ALAT (21.79%) creatinine (60.17%) and HDL cholesterol (223.46%). This dose also significantly reduced MDA levels in the liver (64.6%) and kidneys (52.03%), Lastly, this dose significantly reduced MDA levels in the liver (64.6%) and kidneys (52.03%) but increased SOD, GSH and CAT levels by 5.8, 300 and 227 respectively in the liver of rats. The results of this study justify the use of PROMAX-C in the prevention and treatment of type 2 diabetes and its complications.
Keywords:Oxidative stress; PROMAX-C; MACAPOS; Dexamethasone
Abbreviations:ALAT: Alanine Aminotransferase; ASAT: Aspartate Aminotransferase; CAT: Catalase; GSH: Reduced Glutathione; HSD: Hypercaloric Sucrose Diet; IDF: International Diabetes Federation; MDA: Malondialdehyde; NO: Nitric Oxide; T1DM: Type 1 Diabetes Mellitus; T2DM: Type 2 Diabetes Mellitus; SOD: Superoxide Dismutase, IA: Atherogenic Index.
Introduction
One of the most common diseases in the world is diabetes mellitus. It’s a metabolic disorder characterised by chronic hyperglycaemia leading to impaired insulin signalling, which generates metabolic changes and an inflammatory state that eventually affects the body’s tissues [1]. The number of people with diabetes continues to grow at an alarming rate. According to the International Diabetes Federation (IDF), more than 463 million people worldwide have diabetes and more than 19 million of these are in Africa [2]. Cameroon is no exception, with around 615300 people living with diabetes mellitus [3]. The American Diabetes Association (ADA) has classified diabetes mellitus into Type 1 Diabetes Mellitus (T1DM), Type 2 Diabetes Mellitus (T2DM), gestational diabetes and many other specific types of diabetes [4]. T2DM is much more widespread and is responsible for 90% of the incidence of diabetes mellitus [5,6]. Oxidative stress is closely linked to diabetes mellitus, particularly T2DM, where it very often causes micro and macro angiopathies [7]. Oxidative stress can cause extensive damage to cells, tissues and even organs by altering important biomolecules and cells [8]. The production of high levels of reactive oxygen species causes a significant reduction in antioxidant defence mechanisms, leading to damage to proteins, lipids and DNA with subsequent disruption of cellular function, cell death and subtle changes in intracellular signalling pathways [9]. Consequently, a drug containing both antioxidant and antidiabetic properties would be useful for treating diabetes mellitus and its complications [10].
The management of diabetes mellitus includes lifestyle modifications, pharmacotherapy and nutritional therapy. The treatment of T2DM is based on the use of various oral antidiabetic drugs such as sulfonylureas, biguanides and alpha glucosidase inhibitors [11]. These drugs are often the cause of numerous side effects in diabetic patients, including the risk of hypoglycemic coma, digestive intolerance (nausea, diarrhoea, bloating, abdominal pain), anemia, weight gain, increased risk of cardiac arrest and fracture, reduced absorption of vitamin B12 and folic acid, lactic acidosis, and the risk of tissue anoxia [12-14]. In view of all these side effects, new therapies based on natural products for the management of T2DM are essential [15]. Several plants with antioxidant and antidiabetic effects have already been shown to be effective in the treatment of diabetes mellitus, including extracts of Leucomeris spectabilis leaves, Garcinia kola seeds and Boswellia dalzielii bark [16-18]. Numerous experimental animal models of T2DM have been used to validate the use of new therapies and to elucidate the mechanism(s) of action of the drugs tested [19]. Hypercaloric Sucrose Diet (HSD) followed by administration of Dexamethasone (DMS) has been shown to increase insulin resistance in rats, thereby contributing to the development of T2DM. This was followed by oxidative stress [20]. PROMAX-C is an ethanolic extract of propolis harvested from the hives of honeybees in Cameroon. In addition to diabetes, it’s an Improved Traditional drug used to treat wounds, burns, respiratory and dental infections, stomach ulcers, arterial hypertension, amenorrhoea and gynecological disorders [21]. However, there is a lack of data on the anti-diabetic activity of this drugs.
The aim of this study was therefore to evaluate the antidiabetic and antioxidant effects of PROMAX-C on type 2 diabetes induced by a HDS supplemented with dexamethasone (0.2mg/kg/d).
Material and Methods
Chemicals and reagents
Dexamethasone sodium phosphate (Prost Pharma France) and metformin (Sanofi France), ketamine (Rotexme Tritau Germany) and diazepam (Swiss Parenteral India). One-touch glucometer (Accu-check, Roche Diagnostics, Germany) and commercial kits (Fortress diagnostics, UK) were used in this study.
Animals
The animal used in this study was male Wistar rats (Rattus norvegicus) weighing between 160 and 190g from the animal house of the Faculty of Science at the University of Ngaoundéré (Cameroon), reared under ambient temperature conditions, subjected to a 12/12h light/dark cycle, with free access to water and food of standard composition. Female rats were not included in this study due to variations in their menstrual cycle that could interfere with our results. The experimental protocol is approved by the Institutional Animal Ethics Committee.
PROMAX-C
PROMAX-C is a hydroethanolic extract (70%) of Cameroonian propolis, prepared and stored in 30, 60, 125, 250 and 1000mL bottles and marketed by the Association Bee-Flower-Man of Ngaoundéré (Cameroon) for the treatment of various illnesses such as influenza, coughs, dental infections, angina, wounds, headaches, haemorrhoids, digestive and visual disorders, gastric ulcers, urogenital and respiratory tract infections, menstrual problems, appendicitis, hypertension, heart problems and diabetes.
To determine the doses to be administered, the dosage prescribed by the manufacturer is as follows: Two (2) teaspoons (10ml) of PROMAX-C given to us by Professor Fernand-Nestor Tchuenguem Fohouo were dried in an oven at 40 °C for 48 hours. The dry mass obtained was weighed and used to determine the concentration of PROMAX-C ([PROMAX-C]) using the following formula:
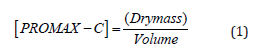
This concentration enabled us to obtain the maximum dose to be used. To obtain the other doses, this maximum dose was diluted by 2,4 and 8 with distilled water. The solutions were administered to rats at a rate of 10mL/kg body weight (bw) [22]. The different doses of PROMAX-C administered to rats were 300.0, 150, 75 and 37.5mg/kg bw.
Evaluation of the effect of PROMAX-C on the Oral Glucose Tolerance Test (OGTT) in normoglycemic rats
This test was carried out to select three doses for the subchronic test of anti-diabetic activity. To do this, 30 normoglycemic rats were fasted for 12h and then divided into 6 groups of 5 animals each. Fasting blood glucose levels were taken at t= -30 min before administration of D-glucose followed by administration of the different treatments. Group 1 received distilled water (10mL/ kg), group 2 metformin (20mg/kg) and groups 3, 4, 5 and 6 were treated with PROMAX-C at doses of 32.5, 75, 150 and 300mg/kg body weight respectively. 30 minutes after administration of the different treatments (t=0min), blood glucose levels were measured and D-glucose (3g/kg bw) was administered orally to all rats. Blood glucose levels were measured using the ACCU-CHEK active glucometer from a small slit in the distal end of the tail of each rat at -30, 0, 30, 60, 120 and 180min after D-glucose treatment.
Evaluation of the anti-diabetic and antioxidant effects of PROMAX-C in diabetic rats
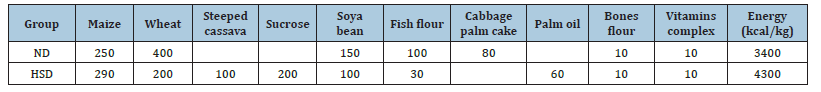
Induction and treatment of type 2 diabetes: The MACAPOS (Maize, Cassava, Palm Oil and sucrose) Hypercaloric Sucrose Diet (HSD) model was formulated following the protocol described by Kamgang et al. [23]. Table 1 shows the composition of the different diets used in our study.
Table 1:Different diet compositions and their nutritional values [23]. ND: Normal (or standard) Diet; HSD: Hypercaloric Sucrose Diet.
Normoglycemic rats were fed by HSD for 30 days. During
this period a glucocorticoid (Dexamethasone 0.2 mg/kg) was
simultaneously administered intraperitoneally every day to
animals in groups 2 to 6 [24,25]. In this study, thirty-five (30) male
rats, including 25 induced and 05 normal rats, were divided into
seven (06) batches of five (05) rats each and treated daily by the
oral route as follows:
a) Group I (Normal control): ND+10ml/kg distilled water
b) Group II (Diabetic control): HSD+0.2mg/kg
Dexamethasone+10ml/kg distilled water
c) Group III (Positif control): HSD+0.2mg/kg
Dexamethasone+20mg/kg Metformin
d) Group IV: HSD+0.2mg/kg Dexamethasone +PROMAX-C 75mg/
kg
e) Group V: HSD+0.2mg/kg Dexamethasone +PROMAX-C 150mg/
kg
f) Group VI: HSD+0.2mg/kg Dexamethasone +PROMAX-C
300mg/kg
All animals were treated in this way for thirty days. During this time, body weight was recorded just before the start of the experiment (week 0), then every week, and blood glucose levels were recorded at the start (day 0), then on day 10, day 20 and day 30. After day 30, the animals were fasted and sacrificed by cervical dislocation following anesthesia by intraperitoneal injection of a mixture of ketamine (50mg/kg bw) and diazepam (10mg/kg bw). Arteriovenous blood collected in dry tubes was centrifuged a 3000rpm for 15 minutes. The collected serum was stored at -20 °C for the determination of biochemical parameters. The liver, kidneys and heart were removed, rinsed in 0.9% saline and weighed to determine relative weight according to the formula:
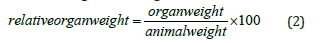
Tris-HCl buffer (50mmol) was used for homogenates (20%w/v) from liver and kidney. Each homogenate was centrifuged at 3000rpm for 15 minutes and the supernatant collected was stored at -20 °C for assessment of oxidative stress parameters.
Biochemical analysis
The lipid profile (cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol), urea, bilirubin and proteins were determined using the Fortress kit (United Kingdom). the activities of the transaminases: Alanine Aminotransferase (ALAT) and Aspartate Aminotransferase (ASAT) were determined using the method of Reitman and Frankel, while the creatinine level was determined using the method of Bartels et al. [26,27]. Protein concentration was determined according to Gornal et al. [28]. Malondialdehyde (MDA) was determined using the procedure of Wills [29], Superoxide Dismutase (SOD) was determined using the method of Misra and Fridovish [30], whereas Reduced Glutathione (GSH) was assessed using the method of Ellman [29-31]. Nitric Oxide (NO) was measured using the method of Fermor et al. [32] and Catalase Activity (CAT) was assessed using the method of Sinaha et al. [33]. The Atherogenic Index (AI) was calculated as follows [34].
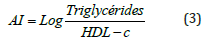
Result
Effect of PROMAX-C on the Oral Glucose Tolerance Test (OGTT) in normal rats
Figure 1.Effect of PR0MAX-C on fasting blood glucose levels in rats fed DHS+ DMS for 30 days. Values are expressed as mean± SEM (n = 5). aP<0.05, cP<0.001: Significant difference from normal control group. βP<0.01, ℽP<0.001: significant difference from diabetic control group.
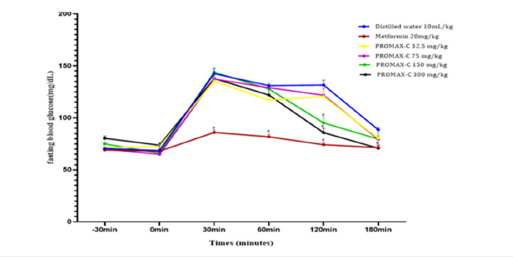
Before administration of D-glucose at times T= -30 and T= 0min, mean blood glucose levels did not vary significantly in the different groups of animals. After administration of D-glucose, blood glucose levels increased significantly (p<0.001) in all groups of rats throughout the treatment period. The glycemic peak was observed at the thirtieth minute (T= 30min) in all animal groups except the metformin-treated group. These blood glucose peaks were 142.6±5.15; 135.2±3.9; 137.4±2.42; 144±4 and 137.8±4.09mg/dL blood glucose respectively for animals in the distilled water, 32.5; 75; 150 and 300mg/kg PROMAX-C treated groups. However, the glycemic peak for metformin was not observed until the sixtieth minute (T=60min) after administration of D-glucose and amounted to 117.2±0.86mg/dL blood glucose. Thereafter, blood glucose levels decreased progressively in all groups until normalization was observed after 180 minutes in all groups of animals. The maximum reduction was observed with the metformin treatment and amounted to 49.93% after 180 minutes. On the other hand, for the groups of animals treated with PMC, the significant (p<0.001) and maximum glycemic reduction was observed with the 300mg/ kg dose and amounted to 34.8% after 180min (Figure 1).
Effect of PROMAX-C on fasting blood glucose levels in rats
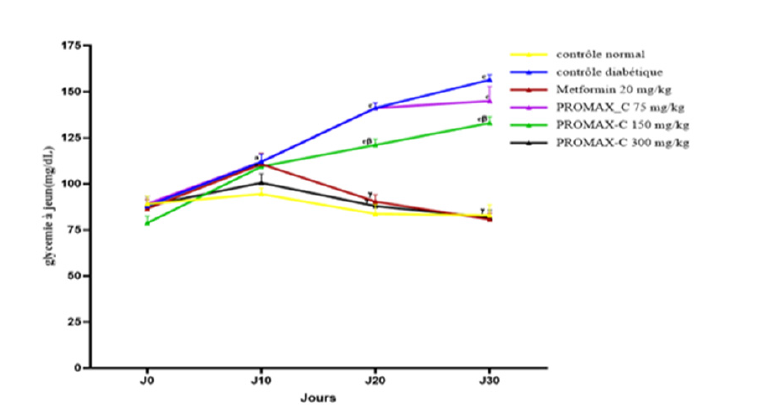
The effect of PROMAX-C on fasting blood glucose levels in DHS+ Dexamethasone-induced rats are shown in Figure 2. This table shows that the blood glucose levels of animals in the diabetic control group showed a significant (P<0.001) and gradual increase throughout the treatment period compared with the normal control. Blood glucose levels rose from 88±5.26mg/dL on day 0 to 156.5±2.75mg/dL on day 30. However, metformin and PROMAX-C administered daily to the animals significantly (P<0.001) prevented the rise in blood glucose levels throughout the treatment period. Blood glucose levels in animals treated daily with metformin fell from 86.6±5.03mg/dL on day 0 to 80.75±3.38mg/dL on day 30. The blood glucose levels of animals treated daily with PROMAX-C at different doses increased between day 0 and day 10 for all doses of PROMAX-C administered. The 300mg/kg PROMAX-C dose showed a significant reduction (P<0.001) in blood glucose from 100.6±4.83 on day 10 to 81.75±4.01mg/dL on day 30.
Figure 2.Effect of PR0MAX-C on fasting blood glucose levels in rats fed DHS+ DMS for 30 days. Values are expressed as mean ± SEM (n=5). aP<0.05, cP<0.001: Significant difference from normal control group. βP<0.01, ℽP<0.001: significant difference from diabetic control group.
Effects of PROMAX-C on body weight and relative organ weights in rats
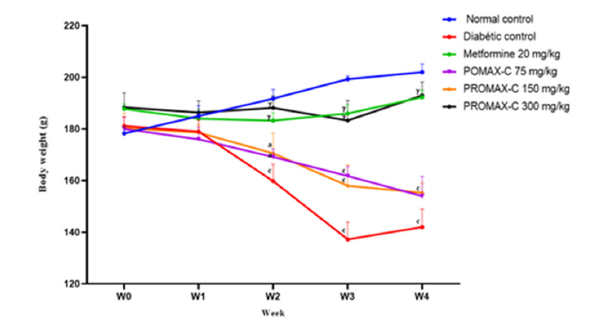
The effect of PROMAX-C on relative organ weights is shown in Table 2. From this table, no significant variation was observed between the relative weights of the heart, liver and kidneys of animals in all groups treated with PROMAX-C extract compared with the diabetic control. The effect of PROMAX-C on body weight is shown in Figure 3. The figure shows that simultaneous administration of the high-calorie sucrose diet and dexamethasone resulted in a significant reduction (p<0.001) in the body weight of animals in the diabetic group compared with normal animals. In the diabetic control group, body weight decreased from 181.2±3.49 g on week 0 to 142±7.02 g on week 4. In the groups treated with metformin and PROMAX-C 300 mg/kg, body weight increased by 2.41% and 2.44% respectively between week 1 (S1) and the last week (S4) of the experiment. Values are expressed as mean ± SEM (n=5). aP<0.05, bP<0.01, cP<0.001. significant difference compared to the normal control group. αP<0.05, βP<0.01, ℽP<0.001. significant difference compared with the diabetic control group.
Table 2:Effects of PMC on body weight and relative organ weight in rats induced with DHS + DMS for 30 days..
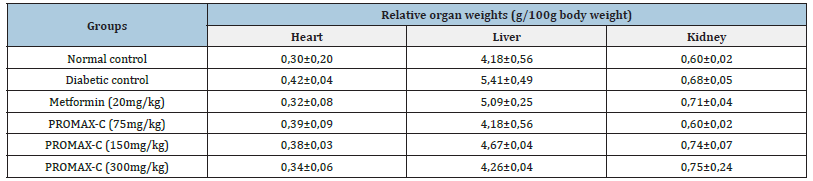
Figure 3.Effect of PROMAX-C on body weight in rats fed DHS+ Dexamethasone for 30 days. Values are expressed as mean ± SEM (n = 5). aP<0.05, bP<0.01, cP<0.001. significant difference compared to the normal control group. αP<0.05, βP<0.01, ℽP<0.001. significant difference compared with the diabetic control group.
Effects of PROMAX-C on lipid profile and atherogenic index in treated rats
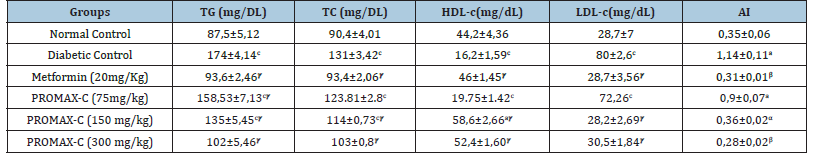
The lipid profile of animals treated with a hypercaloric sucrose diet combined with Dexamethasone is shown in Table 3. It can be seen from this table that in the diabetic control group animals that received a hypercaloric sucrose diet and distilled water, except for HDL cholesterol, the lipid profile was significantly (p<0.001) higher than in the normal control group animals that received the normal diet and distilled water simultaneously. This increase was of the order of 98.86%, 44.91% and 178.75% respectively for triglycerides, total cholesterol and LDL cholesterol. There was also a significant reduction (p<0.001) in this to the HDL cholesterol levels of around 63.35% in animals in the diabetic control group compared with animals in the normal control group. However, the simultaneous daily administration of PROMAX-C at a dose of 300mg/kg to the 20mg/kg dose and the hypercaloric sucrose diet to the animals significantly (p<0.001) prevented the increase in triglyceride, total cholesterol and LDL cholesterol levels by 41.38%, 21.37% and 61.88% respectively. Similarly, simultaneous daily administration of the PROMAX-C significantly (p<0.001) increased HDL cholesterol levels to 223.46%, compared with the diabetic control. Daily administration of metformin (20mg/kg) with the sweetened hypercaloric diet also prevented a significant increase (p<0.001) in triglyceride levels (46.21%), total cholesterol (28.7%) and LDL cholesterol (64.13%) but caused a significant increase in HDL cholesterol levels (183.95%) compared with animals in the diabetic control group. For the atherogenic index, the table shows that the animals in the diabetic control group had a higher index than the animals in the normal control group fed the normal diet and distilled water simultaneously. Administration of PROMAX-C at a dose of 300mg/kg significantly (P<0.01) prevented an increase in the atherogenic index (0.28±0.02) compared with animals in the diabetic control group (1.14±0.11).
Table 3:Effects of PROMAX-C on lipid profile and atherogenic index in rats treated with DHS + Dexamethasone for 30 days.
Effects of PROMAX-C on hepatic, renal and total protein parameters in rats
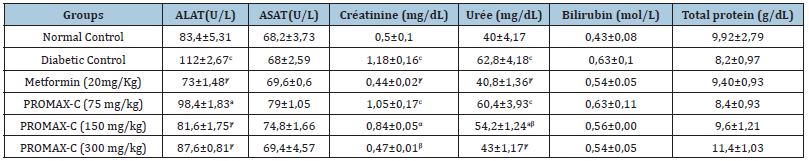
Table 4 shows the effect of PROMAX-C on liver and kidney parameters and total protein levels. It can be seen from this table that in the diabetic control group given the hypercaloric sucrose diet and distilled water, ALAT, creatinine and urea levels were significantly (p<0.001) higher compared to the normal control group given the normal diet and distilled water simultaneously. These values are of the order of 34.29%, 136% and 57% for ALAT, creatinine and urea respectively. Then in However, the simultaneous administration of either PROMAX-C extract at a dose of 300mg/ kg or metformin at a dose of 20mg/kg to the animals significantly (p<0.001) prevented the increase in ALAT, creatinine and urea levels compared with those observed in the diabetic control group.
Table 4:Effects of PROMAX-C on certain liver and kidney parameters in rats treated with DHS + DMS for 30 days.
Effects of PROMAX-C on oxidative stress parameters in rats
The effects of PROMAX-C on oxidative stress parameters (MDA, GSH, SOD, CAT and NO) are shown in Table 5. This table shows that after 30 days of treatment of the animals, the level of MDA increased significantly (P<0.001) in the liver and kidneys of all the animals in the diabetic control group that had received the hypercaloric sucrose diet and distilled water compared with the animals in the normal control group. However, animals given either the reference drug (metformin 20mg/kg) or PROMAX-C (75,150 and 300mg/ kg) simultaneously with the hypercaloric sucrose diet showed a significant (P<0.001) reduction in MDA levels. The percentages observed in the liver were 58.96% and 64.6% respectively for metformin (20 mg/kg) and PROMAX-C (300mg/kg). In the kidneys, the percentages were: 62.33%, 52.03% respectively for metformin (20mg/kg) and PMC (300mg/kg).
Table 5:Effects of PROMAX-C on oxidative stress parameters in rats. MDA (μmol/g of organ), SOD (U/g of organ), GSH (μmol/g of organ), CAT (mM of H2O2 min/g of organ), NO (μmol/g of organ).
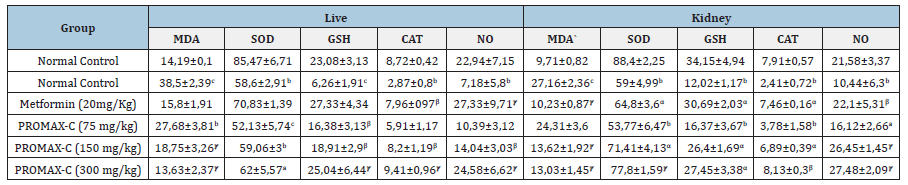
After 30 days of treatment, SOD levels were significantly (P<0.01) lower in the liver (31.45%) and kidneys (33.24%) of animals in the diabetic control group compared with animals in the normal control group given the normal diet and distilled water simultaneously. However, the simultaneous administration of either PROMAX-c or metformin significantly (P<0.001) increased the level of SOD in the kidneys. The percentages observed were: 31.86% and 9.83% for PROMAX-C (300mg/kg) and metformin (20mg/ kg) respectively. GSH levels were significantly lower (P<0.001) in the diabetic control group than in the normal control group. The rates of decline observed were 72.88% and 64.80% in the liver and kidney respectively. Simultaneous daily administration of either PROMAX-C or metformin significantly (P<0.001) increased GSH levels in the liver by 300 and 336.58% for PROMAX-C (300mg/kg) and metformin (20mg/kg) respectively. In the kidneys, this increase was 128.37 and 155.32% respectively for PROMAX-C (300mg/kg) and metformin (20mg/kg) compared with animals in the diabetic control group. For the CAT, we also observed a significant (P<0.01) reduction in CAT activity of 67.09% in the liver and 51.62% in the kidney and in NO of 68.7% in the liver and 51.62% in the kidney in animals in the diabetic control group compared with animals in the normal control group. The 300mg/kg dose of PROMAX-C and metformin (20mg/kg) administered to the animals significantly (P<0.001) increased CAT activity in the liver by 227.87 and 177.35% respectively for PROMAX-C and metformin compared with animals in the diabetic control group.
Discussion
Reducing the growing incidence of type 2 diabetes is a challenge for the global medical system, requiring the development of effective low-toxicity therapeutic approaches. Traditional pharmacopoeia can be used to develop drugs to improve insulin sensitivity, prevent insulin resistance and oxidative stress. The aim of this study was to evaluate the preventive effects of PROMAX-C on T2DM and oxidative stress induced in rats.
The orally induced hyperglycaemia test was carried out to select the different doses for assessing the antidiabetic activity in rats. Doses of 75, 150 and 300mg/kg were the most effective in reducing postprandial glycaemia and will be retained for further research. Phytochemical studies carried out on PROMAX-C revealed the presence of numerous bioactive compounds, including polyphenols, tannins, saponins and flavonoids. These various bioactive compounds are recognised as hypoglycemic agents [35,36]. They act in isolation or synergistically and by different mechanisms to lower blood glucose by stimulating insulin secretion, by mimicking the action of insulin or by inhibiting gluconeogenesis and glycogenolysis [37,38]. PROMAX-C could act via these different mechanisms but also by simultaneously inhibiting intestinal glucose absorption and glucose transporters [39].
The basic mechanism of hyperglycaemia in type 2 diabetics is thought to be due either to excess hepatic glycogenolysis and gluconeogenesis, in addition to reduced glucose utilization by body tissues, or to inhibition of adiponectin gene expression, which promotes insulin resistance through dysfunction of hormone receptors in the liver, muscle and adipose tissue [40-42]. Repeated administration of PROMAX-C and metformin to rats as a preventive treatment for T2DM prevented an increase in blood glucose levels. This anti hyper glycemic effect observed in rats could be explained in part by a reduction in glucose production in the liver and, above all, an improvement in glucose used by the muscles and by activation of the GLUT4 transporters [43]. PROMAX-C, like metformin, would therefore act by an identical or almost similar mechanism to prevent blood sugar levels from rising. PROMAX-C could therefore act at intestinal level by preventing the secretion of digestive juices, which are designed to transform the sweet hypercaloric food into nutrients (glucose, fatty acid, amino acid, etc.) via bioactive compounds such as polyphenols, flavonoids and saponins [44,45].
Induction of T2DM by DHS +Dexamethasone resulted in a significant loss of body weight in diabetic rats compared with normal rats which may be due to loss of muscle and fat mass resulting from excess breakdown of tissue proteins and fatty acids [46]. Glycosuria is known to cause a significant loss of calories for each gram of glucose excreted and presumably this loss leads to significant weight loss despite increased appetite [47]. Rats treated with PROMAX-C and metformin showed an improvement in body weight compared with the diabetic group. These results show a protective effect of the extracts against muscle wasting, probably due to the flavonoids and saponins which stimulate amino acid uptake and protein synthesis, as well as inhibiting proteolysis, resulting in the use of glucose as an energy source [48]. However, this study did not find any significant difference in relative organ weight between the different experimental groups, showing a similarity in results to Tchamadeu et al. [49] who studied the preventive effect of aqueous extract of the stem bark of Pterocarpus soyauxii Taub (Papilionaceae) on insulin resistance and oxidative stress induced by dexamethasone in rats [49].
During the induction of diabetes, dexamethasone stimulates lipolysis and lead to an increase in plasma levels of non-esterified fatty acids capable of inducing insulin resistance. Diet induced hyperglycaemia after 30 days of administration is thought to modify membrane fluidity and facilitate the entry of corticoids into the cell, amplifying their effect [50]. The increase in lipemia in diabetic animals is also thought to be due to the absorption of nutrients from the high-calorie diet during digestion, or to lipolysis following a reduction in the peripheral sensitivity of the animals’ tissues to insulin. The increase in triglycerides leads to an increase in plasma levels of non-esterified fatty acids, which have the potential to induce insulin resistance [51]. The dyslipidemia observed in diabetic animals has led to a considerable increase in the atherogenic index associated with Nitric Oxide (NO) bioavailability, both of which are favourable conditions for the development of cardiovascular disease. NO bioavailability can be used as a predictor of cardiovascular events [52]. The anti-hyper lipidemic effect of PROMAX-C observed in rats may be due to the presence of flavonoids and alkaloids in the drug, indicating its cardioprotective effect [53].
The significant increase in ALAT levels in the blood of animals in the diabetic group compared with those in the normal group would indicate liver damage due to the administration of a hypercaloric sucrose diet combined with dexamethasone. The significant increase in creatinine and urea levels observed in the blood was due to impaired renal function in the diabetic group. Treatment of the animals with PROMAX-C significantly prevented the increase in these parameters, suggesting a protective effect of this extract on these functions. The effect could be due to the reduction or inhibition of amino acid catabolism under the influence of the flavonoids and saponins contained in our traditional drugs. Flavonoids and saponins are involved in improving and repairing liver and kidney function [54,55].
Hyperglycaemia is a well-known cause of elevated levels of free radicals, followed by the production of Reactive Oxygen Species (ROS), which can lead to increased lipid peroxidation, altered antioxidant defenses leading to impaired glucose metabolism [56]. The increase in the level of MDA, a marker of lipid peroxidation, followed by a reduction in the levels of SOD, GSH, CAT and NO in rats in the diabetic group suggests the installation of oxidative stress due to the free radicals produced by DHS associated with DMS. Sub chronic treatment with PROMAX-C reduced MDA and increased SOD, GSH and CAT, which is probably linked to the beneficial effect of the treatments on insulin sensitivity. The antioxidant properties observed in our drug could be due to the presence of the polyphenols and flavonoids it contains, which can scavenge free radicals [57]. Our results agree with those of El Rabey et al. on the anti-diabetic activity of Nigella sativa and Propolis on streptozotocin-induced diabetes in male rats [58].
Conclusion
Oral administration of PROMAX-C improved acute and chronic hyperglycemia in rats by improving dyslipidemia and improved oxidative stress parameters. These results allow us to confirm the use of this drug by the populations of the cities of northern Cameroon and its surroundings to treat diabetes mellitus and its complications.
References
- Boteanu RM, Uyy E, Suica VL, Antohe F (2015) Highmobility group box 1 enhances the inflammatory process in diabetic lung Arch Biochem Biophys 6: 55- 64.
- International Federation of Diabetes (2019) Atlas of Diabetes, (9th edn), Brussels, Belgium.
- International Diabetes Federation (IDF member(s) In Cameroon) (2020) Prevalence of Diabetes in adults, Cameroon.
- American Diabetes Association (2006) Diagnosis and classification of diabetes mellitus. Diabete Care 30(1): 42-47.
- Wild SH, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030: Response to rathman and giani. Diabetes Care 27(10): 1047-1053.
- Cheng D (2005) Prevalence, predisposition and prevention of type II diabetes. Nutrition & Metabolism 2(1): 29.
- Bonnefont-Rousselot D, Beaudeux JL, Thérond P, Peynet J, Legrand A, et al. (2004) Diabetes mellitus oxidative stress and advanced glycation endproducts. French Pharmaceutical Annals 62(3): 147-157.
- Obisike UA, Boisa N, Nwachuku EO (2021) Pomegranate seed extract: A strong antioxidant against benign prostatic hyperplasia induced oxidative stress in albino Wistar rats. J Can Tum Int 11(4): 50‑60.
- Seddon M, Looi YH, Shah AM (2007) Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93(8): 903‑907.
- Yogesha M, Grace NJ, Narendhirakannan RT (2013) Antidiabetic and antioxidant properties of triticum aestivum in streptozotocin-induced diabetic rats. Advances in Pharmacological and Pharmaceutical Sciences (11): 716073.
- Haute Autorité de Santé HAS (2013) Medication strategy for glycemic control in type 2 diabetes, p.
- Andersen SE, Christensen M (2016) Hypoglycemia when adding sulphonyl urea to metformin: a systematic review and Network meta-analysis. Br J Clin Pharmacol 82(5): 1291-302.
- Gomez-Huelgas R, Gomez PF, Rodrugez ML, Formiga F, Puig DM, et al. (2016) Treatment of type 2 diabetes mellitus in elderly patients. Rev Clinica Esp 2018 218(2): 74-88.
- Kancherla V, Elliott JL, Patel BB, Holland NW, Johnson TM, et al. (2017) Long-tern metformin therapy and monitoring for vitamin B12 deficiency among older veterans. J Am Geriatr Soc 65(5): 1061-1066.
- Rekha MB, Bhandare B, Satyanarayana V, Hemamalini MB (2021) Study on antihyperglycemic effect of bromocriptine in dexamethasone induced hyperglycaemic wistar rats. Int J Basic Clin Pharmacol 10(6): 669-703.
- Baiga VP, Kumar K, Yadav S, kumar P, Shukla D et al. (2018) Evaluation of antidiabetic activity of leucomas spectabilis extract in alloxan-induced diabetic rats. Journal of Drug Delivery and Therapeutics 8(5):273-279.
- Nkono Ya, Nkono BL, Rouamba A, Kinyok McJ, Omokolo JGS, et al. (2022) Antidiabetic and antiradical effects of garcinia kola seeds in dexamethasone‑induced hyperglycemic rats. International Journal of Applied and Basic Medical Research 12(3): 203-210.
- Ibrahim AA, Abdussalami SM, Appah J, Umar HA, Muhammad UA, et al. (2023) Evaluation of antihyperglycemic activity of aqueous stem bark extract of Boswellia dalzielii in alloxan-induced diabetic Wistar rats. Future Journal of Pharmaceutical 9: (7).
- Elamin NMH, Fadlalla IMT, Omer SA, Ibrahim HAM (2018) Histopathological alteration in STZ-nicotinamide diabetic rats, a complication of diabetes or a toxicity of STZ. International Journal of Diabetes and Clinical Research 5(3): 1-9
- Ngounou EMD, Mang YD, Dongmo F, Malla OWI, Dongmo SS, et al. (2021) Effect of the aqueous extract of Clerodendrum thomsoniae linn (verbenaceae) leaves on type 2 diabetic wistar rats induced by the MACAPOS1 type diet and dexamethasone. Universal Journal of Pharmaceutical Research 6(3): 9-16.
- Mbawala A, Tchuenguem FFN, Roger D, Millière JB (2009) Spectra of antibacterial activity of propolis (Promax -C) samples from two localities of Adamaoua province (Cameroon). Research Journal of Microbiology 4(4): 150-157.
- Fokam Tagne MA, Noubissi PA, Teddine Tchoblaouna KJ, Fankem GO, Yaouke R, et al. (2021) Effects of hydroethanolic extract of Cameroonian propolis (Promax-c) on castor oil induced diarrhea in mice. World Journal of Advanced Research and Reviews 7(3): 194-203.
- Kamgang R, Mboumi RY, Ndiilé GPRM, Yonkeu J (2005) Cameroon local diet-induced glucose intolerance and dyslipidemia in adult Wistar rat. Diabetes Research and Clinical Practice 69(3): 224-230.
- Adeneye AA, Olangunju JA (2012) Antiperglycemic antihyperlipidemic and cardioprotective profile of bromocriptine, glibenclamide and metformin combination in dexamethasone -induced hyperglycemic rats. Pharmacologia 3(12): 665-671.
- Ogawa A, Johnson JH, Ohneida M, Mc Alister CT, Inman L et al. (1992) Roles of insulin resistance and beta-cell dysfunction on dexamethasone-induced diabetes. Journal of Clinical Investigation 90(2): 497-504.
- Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamate oxaloacetate and glutamic pyruvate transaminase. Am J Clin Pathol 28(1): 56-63.
- Bartels H, Cikes M (1969) Über chromogene der kreatininbestimmung nach JafféChromogens in the creatinine determination of Jaffé. Clin chim Acta 26(1): 1-10.
- Gornall A, Bradwill C, David M (1949) Determination of serum proteins by means of the biuret reaction. Journal of Biology and chemistry 177(2): 751-766.
- Wills E (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99(3): 667-676
- Misra H, Fridovish I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal Of Biological 247(10): 3170-3175.
- Ellman G (1959) Tissue sulfhydryl group. Archives of Biochemistry and Biophysics 82(1): 70-77.
- Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, et al. (2001) The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. Journal of Orthopaedic Research 19(4): 729-737.
- Sinha AK (1972) Colorimetric assay of catalase. Analytical Biochemistry 47(2): 389-394.
- Niroumand S, Khajedaluee M, Khade RM, Abrishami M, Juya M, et al. (2015) Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran 29: 240.
- Bacanli M, Dilsiz SA, Başaran N, Başaran AA (2019) Effects of phytochemicals against diabetes. Adv Food Nutr Res 89: 209-
- Khera N, Bhatia A (2014) Medical plants as natural antidiabetic Agents. Int J Pharm Sci Res 5(3): 713-729.
- Hii CST, Howell SL (1985) Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. Journal of Endocrinology 107(1): 1-8.
- Sakurai H, Kojima Y, Yoshikawa Y, Kawabe K, Yasu H (2002) Antidiabetic vanadium (IV) and zinc (II) complexes. Coordination Chemistry Reviews 226(1-2): 187-198.
- Nistor Baldea LA, Martineau LC, Benhaddou-Andaloussi A, Arnason JT, Lévy EC, et al. (2010) Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the James Bay Cree traditional pharmacopeia. Journal of Ethnopharmacology 132(2): 473-482.
- Maiti R, Jana D, Das UK, Ghosh D (2004) Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J Ethnopharmacol 92(1): 85-91.
- Della Bella E, Buetti-Dinh A, Licandro G, Paras A, Valentina B, et al. (2021) Dexamethasone induces changes in osteogenic differentiation of human mesenchymal stromal cells via SOX9 and PPARG, but Not RUNX2. Int J Mol Sci 22(9): 4785.
- Wego MT, Poualeu SL, Miaffo D, Nchouwet ML, Kamanyi A, et al. (2019) Protective effects of aqueous extract of Baillonella toxisperma stem bark on dexamethasone induced insulin resistance in rats. Adv Pharm Sce 2019: 8075163.
- Itamar Raz MD (2013) Guideline approach to the treatment of patients with newly diagnosed type 2 diabetes. Diab Care 36(4): 139144.
- Mvongo C, Aimé Noubissi P, Kamgang R, Sara Minka Minka C, Mfopa A, et al. (2015) Phytochemical studies and in vitro antioxidant potential of two different extracts of crinum jagus. Int J Pharm Sci Res 6(6): 2354-2359.
- Burdi DK, Qureshi S, Ghanghro AB (2014) An overview of available hypoglycemic triterpenoids and saponins to cure diabetes mellitus. Int J Adv Life Sci 1(3): 119-128.
- Granner DK (1996) Hormones of the gonads. In: Murray RK, Granner DK, Mayes PA, Rodwell VW (Eds.), harper’s Illustrated Biochemistry. McGraw Hill, New York, USA, pp. 566-580.
- Tchamadeu MC, Dzeufiet PDD, Nana P, Blaes N, Girolami JP et al. (2017) Antidiabetic effects of aqueous and dichloromethane/methanol stem bark extracts of Pterocarpus soyauxii Taub (Papilionaceae) on streptozotocin- induced diabetic rats. Pharmacognosy Research 9(1): 80-86.
- Samadi N, Mozaffari-Khosravi H, Rahmanian M, Askarishahi M (2017) Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: A randomized, double-blind clinical trial. J Integr Med 15(2): 124-34.
- Tchamadeu MC, Tsangue YR, Ténézoguang TT, Bogning ZC, Emambo P, et al. (2022) Pterocarpus soyauxii taub (Papilionaceae) aqueous stem bark extract prevents dexamethasone-induced insulin resistance and oxidative stress in rat. Journal of Diseases and Medicinal Plants. 8 (1): 1-12.
- Nobuhiro S, Hideyuki S, Takehide O, Midori F, Hideki K, et al. (2002) Humoral regulation of resistin expression in 3T3-L1 and mouse adipose cells. Diabetes 51(6): 1737-1744.
- Shanmugam S, Ramar S, Ragavendhar K, Ramanathan R, Rajendran K (2009) Plants used as medicine by Paliyar tribes of Shenbagathope in Virudhunagar district of Tamil Nadu. Journal of Economic and Taxonomic Botany 32(4): 922 - 929.
- Rotimi SO, Omotosho OE, Rotimi OA, (2011) Persistence of acidosis in alloxan-induced diabetic rats treated with the juice of Asystasia gangetica leaves. Pharmaco Mag 7(25): 25-30.
- Oršolić N, Sirovina D, Končić ZM, Lacković G, Gregorović G, (2012) Effect of croatian propolis on diabetic nephropathy and liver toxicity in mice. BMC Complementary and Alternative Medicine 12: 117.
- Mamudu EV, Asma’u YO, Babalola AD (2018) Hypocholesterolaemia effect of nauclea latifolia fruit on glucose changes and lipid profile of alloxan induced diabetes in albino rats. International Journal of Advances in Scientific Research and Engineering 4(10): 13-20.
- Babatunde IR, Abdulbasit A, Oladayo MI, Olasile OI, Olamide FR, et al. (2015) Hepatoprotective and pancreatoprotective properties of the ethanolic extract of nigerian Propolis. J Intercult Ethnopharmacol 4(2): 102-108.
- Balasubashini MS, Rukkumani R, Viswanathan P, Menon VP (2004) Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother Res Int J Devoted Pharmacol. Toxicol Eval Nat Prod Deriv 18(4): 310-340.
- Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD (2014) Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Science and Technology 34(1): 16-23.
- El Rabey HA, Al-Seeni MN, and Bakhashwain AS (2016) The antidiabetic activity of nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evidence-Based Complementary and Alternative Medicine 2017: 5439645.
© 2023 Didier Beyssiri. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






