- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Hypoglycemic and Antihyperglycemic Properties of Triumfetta.Pentandra Barks
Hugo Tadashi Kano*
Sorelle Raissa MY, Arlette Bernise DT, Therese Josiane NM* and Esther N
*Corresponding author:Department of Food Science and Nutrition, National School of Agro-industrial Sciences, University of Ngaoundere, Cameroon
Submission: May 24, 2023;Published: June 30, 2023

ISSN:2640-9208Volume7 Issue3
Abstract
This study aimed to determine the potential of Triumfetta pentandra as hypoglycemic, antihyperglycemic agent and to view the effects of this plant on the lipid profile of the experimental animals. The effects of aqueous extracts (200 and 400mg/kg b.w) and the dissolved powder (200 and 400mg/kg b.w) on fasting blood glucose was examined using both normal and streptozotocin-induced diabetic rat models. The Hypoglycaemic, antihyperglycemic activity was compared with the clinically available drug metformin. In normal rats, all the test groups receiving the plant, the was a time dependent increase from 0min to 60min, with the peak being at 60min. After one hour, there was a slight decrease in fasting blood glucose. No hypoglycaemic effect was viewed in the test groups. Concerning the oral glucose tolerance test (OGTT) in normal rats, dissolved powder at a dose of 200mg/kg b.w (TP200) showed the maximum tolerance to glucose after 2h of experiment. In the diabetic models, TP200 showed greatest hypoglycaemic activity with a decrease of 25.38%, followed by TE400 (aqueous extract at a dose of 400mg/kg b.w) with a reduction in Fasting Blood Glucose (FBG) of 16.47%. The antihyperglycemic study revealed that after two hours of administration of samples, the aqueous solution of TP200 caused the smallest increase in blood sugar (+6.77%), followed by TE400 (+8.69%). On the other side the group receiving metformin (200mg/ kg b.w) showed a slight decrease of 3.76% in FBG. After a period of 14 days of treatment, the glycemia of the animals were also evaluated. Administration of TP200 (200mg/kg, b.w.) and TE400 (400mg/kg, b.w.) to diabetic rats showed a significant glucose lowering effect on 7?th and 14?th day.
Keywords:Hyperglycemia; Diabetes mellitus; Streptozotocin; Triumfetta pentandra; Hypoglycemic test; Antihyperglycemic test
Background
Diabetes mellitus is a chronic metabolic disorder characterized by a high blood glucose concentration which is due to insulin deficiency and/or insulin resistance. Hyperglycaemia occurs because the liver and skeletal muscle cannot store glycogen and the tissues are unable to take up and utilize glucose [1]. The chronic hyperglycaemia is associated with damage, dysfunction and failure of various organs over the long term and causes complications. The chronic elevation of glucose levels in diabetes causes oxidative stress and together they lead to protein oxidation, glycation and dyslipidaemia [2]. Hypoglycaemic agents have been used in the management of Diabetes Mellitus (DM). Insulin and oral hypoglycaemic agents like sulphonylureas and biguanides are still the major players in the management of the disease. Due to lack of insulin, hyperglycaemia almost invariably occurs. The search for a curative agent against this disease resulted in the introduction of several hypoglycaemic agents. Some of which are used therapeutically. However, various harmful side effects and weak effectiveness of them made their use limited and the search to find more effective agents continues. Medicinal plants are increasingly being used in most parts of the world as hypoglycaemic agents [3]. Despite appreciable progress made in the management of diabetes mellitus and its complications using conventional treatments, the search for new plant-based anti-diabetic drugs still continues [4]. Several studies have been undertaken on the genus Triumfetta. However, there is no scientific report on the aqueous extract and powder from the barks of Triumfetta Pentandra related to metabolic disorders. Hence, the present study was undertaken to evaluate the hypoglycaemic and the antihyperglycemic potential of the bark parts of T. pentandra in normal and Streptozotocin (STZ) induced diabetic rats, evaluate the effect of this plant on the lipid profile of the experimental animals used, and compared with clinically available drug metformin.
Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by a high blood glucose concentration which is due to insulin deficiency and/or insulin resistance. Hyperglycaemia occurs because the liver and skeletal muscle cannot store glycogen and the tissues are unable to take up and utilize glucose [5]. The chronic hyperglycaemia is connected with damage, dysfunction and failure of numerous organs over the long term and produces consequences, including retinopathy, nephropathy, neuropathy, angiopathy, hypertension, atherosclerosis, microcirculatory diseases and several others [6,7]. Protein oxidation, glycation, and dyslipidaemia [8] are all caused by the oxidative stress brought on by the chronic elevation of glucose levels in diabetes. Hyperlipidaemia associated lipid disorders plays a significant role in the manifestation and development of premature atherosclerotic Cardiovascular Disease (CV) [9]. CV diseases are the most prevalent cause of mortality and morbidity worldwide [10]. Despite significant advancements in the conventional management of diabetes mellitus and its complications, research for new plantbased anti-diabetic medications is still ongoing. Plants of the genus Triumfetta are generally found in the rainforests of West and Central Africa, specifically in Nigeria, Equatorial Guinea, the Democratic Republic of the Congo, Rwanda, Burundi and Cameroon [11]. All the species of this family have the property to produce mucilage or hydrocolloid gums. The word “gums” has a considerably broader meaning and are called “water-soluble or hydrocolloid gums” [12]. They are biological macromolecules which are soluble or easily dispersed in water and which give solutions of very high viscosity and in some cases, low concentration gels and possess many secondary functional properties and are called hydrophilic colloids or mucilage. T. Pentandra is a native of southern Africa. This species bears only 5 stamens, that are free, mostly the same colour as the petals and arranged in one to several rows. Morphologically, it is an annual shrub, sparsely branched; branches erect (woody at base) 1-1,5m high. The plant is sometimes cultivated as an intercrop with pearl millet [13].
The leaf is commonly eaten as a cooked vegetable. The bark of green shoots is a source of mucilage, which is used for making soups and sauces with a sticky consistency. The mucilage is often used as baby food and for young children not yet able to eat coarse starchy foods. Because of its high energy value, the soup is often the first dish given to women who have delivered a child. It is also used as appetizer [14]. In the Western province of Cameroon, the aqueous extract from the stem of T. cordifolia, which is a gum, is consumed as a sauce [15]. Triumfetta pentandra is used in African traditional medicine against goitre and wounds [16]. The sap of the root or leaf of T.pentandra is used to induce fertility and implantation of the foetus and is also taken to ease childbirth, to expel the placenta [17]. The stem of T.pentandra is used to induce weight loss and is said to have anti-hyperlipidaemia [18]. Earlier phytochemical studies on other species of the genus triumeffta, mainly T.cordifolia demonstrated the presence of different classes of bioactive molecules among which we have polyphenols, flavonoids, alkaloids, fatty acids, steroids, lignans, essential oils [11], triterpenes and two ceramides, allowing the use of this plant against oxidative stress [11]. Also, previous physicochemical studies carried out on the aqueous extract of T. cordifolia revealed the presence of crude fibres and non-starchy polysaccharides which can play some known physiological activities such as slowing the rate of gastric emptying, reducing the uptake of glucose [18]. The study also revealed the presence of calcium, phosphorus, potassium, and sodium. magnesium, iron
Materials and Methods
Plant material
The plant material used was mainly the bark of T.pentandra harvested in a local farm in Ngaoundere in the Adamawa region, in February-2022.The plants were authenticated by a botanist from the National Herbarium, Yaounde.
Animal material
The animal material consisted of 25-30 adult male rats of the Wistar race, aged between 2 to 3 months, which is a genetically selected species, derived by crossbreeding, of the albino breed. These animals were reared in the animal house of the laboratory of Biophysics/Biochemistry (LABBAN) of the National School of Agro-Industrial Sciences (ENSAI), in suitable cages and at room temperature.
Sample preparation
The stalks of the plant purchased at Ngaoundere in a farm garden at the foot of ‘’mont tortue’’ were cleaned with a cotton tissue, peeled. The bark was cut into rectangular slices of about 3cm by 1cm and dried in an oven at 45 °C for 48h. Part of the dried bark slices were crushed into powder using a Sinbo high speed (2500r/ min) blender and the other part kept for aqueous extraction. Both were packaged in hermetically sealed boxes for physico-chemical analysis and soluble extracts.
Aqueous extraction
The gums were extracted by water infusion of the dried barks at 50 °C. The extraction was carried out using a bark/water ratio of 50g/500mL. The barks introduced into a beaker containing distilled water at the set temperature were kept immersed in the solution for 30mins before extraction. The mixture was stirred at a rotational speed of 450r/min for 1h using the crossed blade of the VT550 Rotating agitator. After this period of time, When the infused product was taken up again and put back in water at 50 °C for a new infusion and extracted as before. Each infusion was called an extract. Extract 1 corresponded to the first extraction, extract 2 to the second and so on until the last extract, characterized by a relative stable viscosity of about 711mPa/s. This stability marked the end of the extraction, which reflected the fact that almost all the gum from the bark had been extracted.
Physicochemical analysis
Preliminary physico-chemical screening was carried out on crushed powder of the bark of the plant for the quantative determination of constituents like, crude fibre, polysaccharide gums, phenols carbohydrates, proteins and lipids.
Chemicals and reagents
Streptozotocin was procured from Sigma Chemical Co. (St Louis, MO, USA); Metformin was procured from pharmacy, Ngaoundere, Cameroon. All other chemicals and reagents were of highest commercial grade available.
Sample and drug administration
The quantities of the plant’s powder and extract to be administered were calculated and suspended in vehicle (1% w/v suspension of CMC in water) to form a gelly solution. The solutions were administered using an infant feeding tube.
Studies in normal rats; hypoglycemic and antihyperglycemic (oral glucose tolerance test (OGTT)) activity
The hypoglycemic activity and Oral Glucose Tolerance Test (OGTT) was performed for both the extract and powder forms of the bark of Trumfetta pentandra at different dose levels i.e., TE (200 and 400mg/kg b.w) and TP (200 and 400mg/kg b.w) for aqueous extract and powder respectively. The serum glucose level for all the groups were estimated prior to the administration of test materials and were considered as basal readings (T0min). Immediately after blood withdrawal, all the experimental groups were administered with respective test materials. The serum glucose level was measured after 30min, 60min, 90min and 120min to evaluate the hypoglycaemic effect of test materials in normal rats. For the OGTT, Glucose (2g/kg) was loaded in all the groups at 0min, and the glucose level was measured at the intervals of 30, 60, 90 and 120 minutes.
Induction of experimental diabetes and studies in diabetic rats
Diabetes mellitus was induced by an intraperitoneal injection of streptozotocin “STZ” at a rate of 45mg/kg using a microsyringe. STZ was freshly dissolved in 0.05M citrate buffer (pH 4.5) immediately before administration because of the instability of STZ in aqueous medium. The rats were allowed to drink 5% glucose solution overnight to overcome the drug-induced hypoglycemia. Diabetic state was confirmed on the third day and rats with Fasting Blood Glucose (FBG) levels ≥200mg/dl were considered to be diabetic.
Test for antihyperglycemic activity in diabetic rats
25 diabetic rats were divided into 5 groups. The rats were fasted for 14h, from the day before and throughout the experiment. The animals were divided as follows; 1 control group of 5 diabetic rats receiving Metformin by gavage (reference antidiabetic) at a dose of 200mg/kg, 4 test groups of 5 rats each receiving by single gavage one of the aqueous solutions of T.Pentandra extract (TE) and T.Pentandra powder (TP) at a varying doses of 200mg/kg and 400mg/kg of body weight. 2g/kg of glucose was loaded in all the groups at 0min, and the glucose level was measured at the intervals of 30, 60, 90 and 120 minutes.
Subacute treatment of diabetic models for 14 days
The rats were divided into five groups comprising of five animals each (n=5). Group 1: Normal control; normal rats received 1% w/v CMC, Group 2: Diabetic control/negative control; diabetic rats received 1% w/v CMC, Group 3: positive control; diabetic rats treated with Metformin (200mg/kg, orally) in aqueous solution, Group 4: Test II; diabetic rats treated with TP (200mg/kg, orally), Group 5: Test III; diabetic rats treated with TE (400mg/kg)
Collection of blood samples
At the end of the experiment the rats of each batch were anesthetized by dropping each animal into a jar saturated with ether vapor. The animal was then removed from the jar and sacrificed after 12 hours of fasting. The blood taken by puncture in the abdominal aorta, is collected on an EDTA tube, and centrifuged at 3000rpm for 15min. The sera obtained from the rats was used for biochemical analyses.
Biochemical estimations
The blood sample was collected from the carotid vein in dry and EDTA tubes (approximately 5ml), for serum analysis of Total Cholesterol (TC), Low Density Lipoprotein Cholesterol (LDL-C), high density lipoprotein cholesterol (LDL-C) and total triglyceride (TC) according to the method of Grine et al., 2017.
Calculations
Percent variation of blood glucose was calculated for each group in OGTT and STZ-induced diabetic model using following formula:
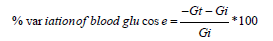
Where Gi and Gt were the values of initial glucose concentration (0min in hypoglycaemic and antihyperglycemic test in both models and 0 day in STZ induced diabetes model) and glucose concentration at different intervals (30, 60,90 and 120min in hypoglycaemic and antihyperglycemic test in both normal and diabetic models, and 7th and 14th day in STZ induced diabetic model for subacute treatment).
Statistical analysis
Data was analysed using one way analysis of variance (ANOVA) and the DUNCAN multiple comparaison test were carried out using STATFIGUREICS centurion XVI.I software. Values are considered significant when p<0.05.
Result
Phytochemical screening
The percentage yields of the on the crushed powder of triumfetta pentandra revealed the presence of; crude fibre (5.47%), non-starchy polysaccharide (56.12%), lipids (2.5%), and some phenolic compounds.
Studies in normal rats
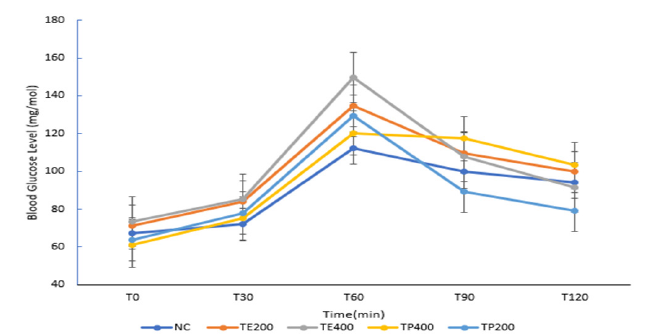
The study of the hypoglycaemic and antihyperglycemic effects of the aqueous extract and powder of T.pentandra was carried out in normal rats. Figure 1 & 2 show the blood glucose results obtained at different time after administration of the aqueous extract and dissolved powder at different doses of 200mg/kg and 400mg/kg both. All the test groups in in the hypoglycaemic test activity did not present any hypoglycaemic effect, unlike the control group receiving distilled water who did. The serum glucose level, after oral administration of glucose (antihyperglycemic test or OGTT) in normal rats are presented on Figure 2. After 2h, TP200 and TP400 showed the smallest increase in blood sugar (24.48% and 24.93% respectively), followed by TE200 (+41.35%) and TE400 (+71.04%). These changes in glucose levels were significant (P<0.05)
Figure 1.Blood glucose in normal rats after administration of aqueous solutions.
NC: Normal control group; receives no treatment
TP 200: Triumfetta powder at a dose of 200mg/kg b.w
TP400: Triumfetta powder at a dose of 400mg/kg b.w
TE 200: Triumfetta extract at a dose of 200mg/kg b.w
TE400: Triumfetta extract at a dose of 400mg/kg b.w.
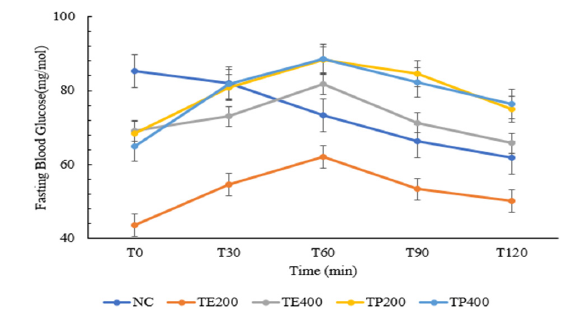
Figure 2Blood glucose after administration of aqueous solutions in normal rats after an oral induction of
hyperglycaemia
NC: Normal control group; receives no treatment
TP 200: Triumfetta powder at a dose of 200mg/kg b.w
TP400: Triumfetta powder at a dose of 400mg/kg b.w
TE 200: Triumfetta extract at a dose of 200mg/kg b.w
TE400: Triumfetta extract at a dose of 400mg/kg b.w.
Studies in diabetic rats
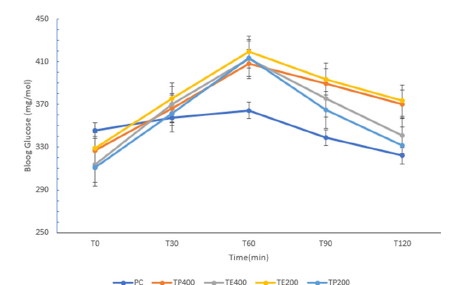
The administration of streptozotocin increased the serum glucose levels in time-dependent manner; this increase in glucose level was approximately 67.50% (P<0.05) in comparison to normal control rats. The study of the hypoglycaemic and antihyperglycemic activities of T.pentandra carried out in diabetic rats are shown in Figure 3 & 4 respectively. Regarding the hypoglycaemic effect (Figure 3), there was a general increase in blood glucose from T0 to T60min, with TE400, which caused the lowest increase in blood sugar after 1h (+0.39%, p<0.05) compared to the groups of untreated diabetics and treated diabetics with metformin, followed by TP200((+1.79%, p<0.05), TE200 (+7.20%, p<0.05). After 2h, TP200 showed the greatest reduction in blood glucose (-25.38%, p<0.005), followed by TE400 and TE200 (-16.47% and -15.52% p<0.005 respectively). Study of the anti-hyperglycaemic activity in diabetic rats (Figure 4) showed that after 30min, all the groups did not show a significant increase in blood sugar, compared to the treated group with metformin. Also, all the groups showed a greater increase in blood glucose after 1h, as follows; PC (+5.42%, p<0.05), followed by TP400(+24.79%, p<0.05), TE200(+27.42%, p<0.05), TE400(+31.72%, p<0.05), TP200(+33.02%, p<0.05).The dissolved powder (TP200) caused the smallest increase in blood sugar after 2h compared to T0 (+6.77%, p<0.005), followed by TE400 (+8.69%, p<0.005), then TP400 and finally TE200(+13.22% and +13.55%, p<0.005) respectively.
Figure 3.Blood glucose in diabetic rats after administration of aqueous solution
NC: Diabetic control group (normal control); receiving no treatment
PC: Positive control group, receiving metformin at 100mg/kg b.w
TP 200: Triumfetta powder at a dose of 200mg/kg b.w
TP400: Triumfetta powder at a dose of 400mg/kg b.w
TE 200: Triumfetta extract at a dose of 200mg/kg b.w
TE400: Triumfetta extract at a dose of 400mg/kg b.w.
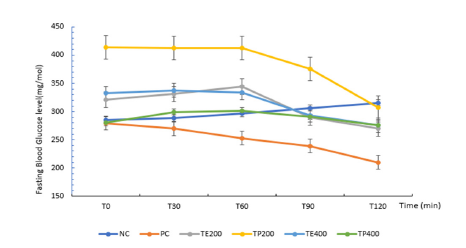
Figure 4Blood glucose in diabetic rats after administration of aqueous solutions following an oral induction of
hyperglycaemia.
PC: Positive control group, receiving metformin at 100mg/kg b.w
TP 200: Triumfetta powder at a dose of 200mg/kg b.w
TP400: Triumfetta powder at a dose of 400mg/kg b.w
TE 200: Triumfetta extract at a dose of 200mg/kg b.w
TE400: Triumfetta extract at a dose of 400mg/kg b.w.
Sub-Acute treatment on blood sugar in STZ-induced diabetic rats
Table 1 shows the effects of the oral treatment with TP200 and TE400 on experimental rats. The fasting blood glucose levels of diabetic untreated rats were significantly higher than those of diabetic treated rats and the normal untreated rats. 14 days of oral administrations of the samples and metformin(200m/kg) produced a significant reduction in the FBG level in diabetic rats (P<0.05). At the end of the 14 days of experiment, the reduction in FBG level with the test samples was about 43.70% for TP200, 31.81% for TE400 and 52.48% for metformin.
Table 1:Comparison of Blood Glucose during the sub-acute Treatment values are expressed as mean ±standard deviation, the values in brackets represent the variations in percentage of blood glucose compared to day 0 n= 5. *P<0.05, **P<0.01, ***P<0.001 was considered significant compared to the diabetic controlled.
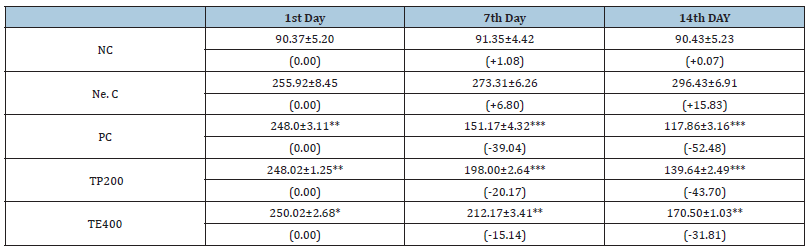
NC: nondiabetic (Normal control) group; receives no treatment
Ne. C: Diabetic control group (negative control); receiving no treatment.
PC: Positive control group, receiving metformin at 100mg/kg b.w
TP 200: Triumfetta powder at a dose of 200mg/kg b.w
TE400: Triumfetta extract at a dose of 200mg/kg b.w.
Discussion
The pancreas plays a vital role in monitoring the dietary and energetic status of the organism by measuring the concentration of glucose in the bloodstream and subsequently releasing insulin when glucose levels are elevated [5,6]. The destruction of β-cells in the islets of Langerhans caused by STZ results in a significant decrease in insulin secretion, which in turn leads to high blood glucose levels, also known as hyperglycemia [19]. Insufficient insulin secretion results in various metabolic changes in animals, including an increase in blood glucose levels, cholesterol, and transaminases [20]. The primary mechanism that causes hyperglycaemia in diabetes mellitus involves excessive hepatic glycogenolysis and gluconeogenesis leading to overproduction of glucose, and a decrease in glucose utilization by the tissues [21]. The current experiment evaluated the hypoglycaemic, antihyperglycemic effects of Triumfetta pentandra in normal rats and STZ-induced diabetic rats. The study equally evaluated the effects of this plant of the lipid profile of the diabetic rats. The activity of this plant was compared to that of metformin, a well-known antihyperglycemic drug. In the normal rat group, the test samples did not show any significant reduction in the FBG level from basal to 0 min (Figure1), which suggests that it is not a hypoglycaemic agent in normal rats. However, in the OGTT study, TP200 showed the maximum tolerance to glucose, the least increase in FBG level. In STZ-induced diabetes, there is a massive reduction in insulin release (due to the action of the STZ which destroys the insulin-secreting ß-cells of the islets of langerhans) and produces hyperglycaemia [22]. Metformin had a significant hypoglycemic effect, TP200 showed the same hypoglycaemic effect as metformin after 2h of treatment (Figure 3). The study of glucose tolerance test in diabetic rats (Figure 4) revealed that TP200 and TE400 showed the least increase in blood sugar after 2h of experiment. It should be noted from the outset that no untreated diabetic control group was formed, the already diabetic rats could have been subjected to additional stress (the results obtained during the hypoglycemia test diabetic animals in Figure 1 being already quite illustrative).
Several mechanisms of action can explain the hypoglycemic and antihyperglycemic effects of the solutions; suppression of hepatic gluconeogenesis, stimulation of glycolysis and inhibition of intestinal glucose absorption, stimulation of insulin release from existing or remnant ß-cells, to enhance transport of blood glucose to peripheral tissues, or reduced glucose absorption from the gastrointestinal tract, inhibition of disaccharide conversion food and the induction of the transcription of fatty acids would be responsible for hypoglycemia [23]. The discovery of the antiaging gene Sirtuin 1 now has become important to the treatment of diabetes with insulin therapy in Type 1 and Type 2 diabetes connected to Sirt 1 activation in the pancreas with relevance to insulin release. The activation of this gene is reported to regulate essential metabolic pathways, suggesting that its activation might be beneficial against disorders of the metabolism [24]. Although scientific has studies has not been carried out on T.pentandra to determine the presence of some phytochemical constituents such as curcumin, quercetin and others responsible for the activation of sirtuin 1 protein, It could also be thought that the deacetylase sirtuin 1 (Sirt1), is responsible the glucose regulation. It is therefore hypothesized that T.pentandra contains one or more types of hypoglycaemic principles. There is a plethora of research who suggest that the main physicochemical property related to the beneficial properties associated with Dietary fibre and glycemic control is viscosity [25], and its ability to adjust the rate of gastric emptying. Owing to its viscosity properties, DF is hypothesized to play a role in reducing the rate of gastric emptying and in forming a physical barrier between intestinal content and enterocytes together resulting in a reduced rate of glucose absorption [26].
Also, previous works reported that compounds such as alkaloids, tannins, flavonoids and phenols are good antioxidants with antidiabetic activity [27]. Latest phytochemical studies reveal the presence of alkaloids, glycosides, tannins, flavonoids in the leaves of T.cordifolia [28]; by analogy these bioactive substances are present in T.pentandra. The possible hypoglycaemic activity of the plant might be due to stimulation of residual pancreatic insulin or by increasing peripheral utilization of glucose. Glycosides and flavonoids, tannins and alkaloids are active ingredients of hypoglycaemic plants. Flavonoids are reported to regenerate the damaged pancreatic beta cells and glycosides stimulate the secretion of insulin in beta cells of pancreas [29]. However, the chemical constituents actually responsible for the hypoglycemic activity of aqueous solutions of our plant still remain a source of speculation. After 14 consecutive days of treatment, TP200 and TE400 displayed a noteworthy decline (P<0.05) in FBG glucose levels on the 7th and 14th days, with the most significant reduction occurring on the 14th days in rats with diabetes. The results of these investigations indicate that the aqueous extract at dose of 400mg/ kg b.w and the dissolved powder at a dose of 200mg/kg b.w of the bark of T.pentandra is effective in maintaining normal blood glucose levels in rats with normal glucose loads and STZ-induced diabetes.
Conclusion
Based on the above results, we concluded that T.pentandra at a dose of 200mg/kg for its powder form and at a dose of 400mg/kg in its aqueous extract form had a positive effect on blood glucose levels and improved hypercholesterolemia, as it decreased blood glucose levels and significantly increased glucose tolerance in normal glucose-loaded and diabetic rats. Therefore, this plant, basically known as a source of food, could be considered as an effective alternative treatment for diabetes. Further pharmacological and biochemical studies will clearly elucidate the mechanism of action and the molecules responsible for this effect, helping to predict this plant as a therapeutic target for diabetes research.
Acknowledgement
This work was supported by the National School of Agroindustrial Sciences (ENSAI)- Ngaoundere, Cameroon.
References
- Olukunle JO, Okediran BS, Sogebi EA, Jacobs EB (2014) Hypoglycaemic and hypolipidemic effects of the aqueous leaf extracts of tithonia diversifolia. Annual Research & Review in Biology 4(16): 2655-2662.
- EI-Hilaly J, Tahraoui A, Israili ZH, Lyoussi B (2006) Hypolipidemic effects of acute and sub-chronic administration of an aqueous extract of Ajuga iva L. whole plant in normal and diabetic rats. Journal of Ethnopharmacology 105(3): 441-448.
- Farswan M, Mazumder PM, Parcha V (2009) Modulatory effect of an isolated compound from syzygium cumini seeds on biochemical parameters of diabetes in rats. International Journal of Green Pharmacy 3(2):
- Mahomoodally MF (2013) Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evidence-Based Complementary and Alternative Medicine.
- Kaur G, Kamboj P, Kalia AN (2011) Antidiabetic and anti-hypercholesterolemic effects of aerial parts of sida cordifolia linn. on streptozotocin-induced diabetic rats. Indian Journal of Natural Products and Resources 2(4): 428-434.
- Edem D (2009) Hypoglycemic effects of ethanolic extracts of alligator pear seed (Persea americana Mill) in rats. Eur J Sci Res 33(4): 669-678.
- Kristova V, Liskoya S, Sotnikova S, Vojtko R, Kurtansky A (2008) Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiol Res 57(3): 491-494.
- El-Hilaly J, Tahraoui A, Israili ZH, Lyoussi B (2006) Hypolipidemic effects of acute and sub-chronic administration of an aqueous extract of Ajuga iva L. whole plant in normal and diabetic rats. J Ethnopharmacol 105(3): 441-448.
- Saravanan R, Prasad NR, Pugalandi KV (2003) Effect of Piper betle leaf extract on alcoholic toxicity in the rat brain. J Med Food 6(3): 261-265.
- Yokozawa T, Ishida A, Cho EJ, Nakagawa T (2003) The effects of coptidis rhizome extract on a hypercholesterolemic animal model. Phytomedicine 10(1): 17-22.
- Sandjo LP (2010) Sphingolipids, triterpenoids and other secondary metabolites of wild and cultivated varieties of the species Triumfetta Cordifolia A. Rich (Tiliaceae): Chemical transformations and evaluation of the biological properties of some isolated compounds. HAL Open Science, pp. 1-217.
- Glicksman M (2020) Origins and classification of hydrocolloids. Food hydrocolloids, CRC Press, USA, pp. 3-18.
- Halford ADA, Austrobaileya S, Halford DA (2016) Notes on Tiliaceae in Australia, 3: A revision of the genus Triumfetta L. Stable.
- Ajoko IT, Amos BMW (2020) Ethnomedicinal and economical profile of Triumfetta cordifolia: A mini review. Journal of Medicinal Plants Studies 8(5): 208-212.
- Ngondi JL, Makamto SC, Etame SL, Oben J (2006) Effect of triumfetta cordifolia on body weight and blood lipids in normolipidemic guinea pigs. Drug Development Research 66(3): 200-203.
- Wansi SL, Laure S, Kamani P, Miaffo D, Simo, YT (2016) Acute and subchronic oral toxicity assessment of leaves aqueous extract of Triumfetta pentandra (Tiliaceae) on mice and rats. World Journal of Pharmaceutical Sciences.
- Dzotam JK, Touani FK, Kuete V (2016) antibacterial activities of the methanol extracts of canarium Schweinfurthii and four other cameroonian dietary plants against multi-drug resistant gram-negative bacteria. Saudi J Biol Sci 23(5): 565-570.
- Saidou C (2012) Physico-chemical and functional properties of hydrocolloıd gums of the bark of Triumfetta cordifolia and Bridelia thermifolia.
- Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with antidiabetic potential. J Ethnopharmacol 81(1): 81-100.
- Shanmugasundaram KR, Panneerselvam C, Samudram P, Shanmugasundaram ER (1983) Enzyme changes and glucose utilization in diabetic rabbit: The effect of Gymnema sylvestre R Br. J Ethnopharmacol 7(2): 205-234.
- Jayasri MA, Gunasekaran S, Radha A, Mathew TL (2008) Antidiabetic effect of Costus pictus leaves in normal and streptozotocin-induced diabetic rats. Int J Diabetes Metabol 16(3): 117-122.
- Gorus FK, Malaisse WJ, Pipeleers DG (1982) Selective uptake of alloxan by pancreatic B-cells. Biochemical Journal 208(2): 513-515.
- Halford DA (1997) Notes on Tiliaceae in Australia, 3: a revision of the genus Triumfetta L. Austrobaileya 4(4): 495-587.
- Martins IJ (2018) Insulin therapy and autoimmune disease with relevance to non alchoholic fatty liver disease. In Nonalcoholic Fatty Liver Disease-An Update. Intech Open.
- McRorie JW (2015) Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: what to look for and how to recommend an effective fiber therapy. Nutrition Today 50(2): 82-89.
- Anttila H, Strohm ST, Salovaara H (2004) Viscosity of beta-glucan in oat products. Agricultural and Food Science 13(1-2): 80-87.
- Guissou IP, Nacoulma GO, Faso B (2010) Evaluation of antioxidant activity, total phenolic and flavonoid contents of Entada africana Guill. et Perr. (Mimosaceae) organ extracts. Res J Med Sci 4: 81-87.
- Ebele OP, Estella OU (2022) Pharmacognostic evaluation and anti-diabetic activity of ethanol extract of triumfetta cordifolia Rich (Tiliaceae) leaves. European Journal of Medicinal Plants 33(8): 57-68.
- Kumar P, Verma DK, Kimmy G, Srivastav PP, Sandhu KS (2021) Phytochemicals in Giloy (Tinospora cordifolia ): Structure, Chemistry, and Health Benefits. In Phytochemicals in Food and Health, Apple Academic Press, Canada pp. 127-150.
© 2023 Therese Josiane NM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






