- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Synthesis, Application and Color Evaluation of Food Dyes Intercalated on Layered Zinc Hydroxide Salt in Cupcakes
Araújo MA1, Kalschne DL2*, Pauli NM1, Bittencourt PRS1, Rocha JCD1, Giona RM1, Canan C2 and Cursino ACT1
1Department of Chemistry, Federal Technological University of Paraná, Brazil
2Department of Food, Federal Technological University of Paraná, Brazil
*Corresponding author:Daneysa Lahis Kalschne, Federal Technological University of Paraná, Brazil Avenue, 4232, Postal Code 85884-000, PO box 271, Medianeira, Paraná, Brazil
Submission: June 05, 2023;Published: June 26, 2023

ISSN:2640-9208Volume7 Issue3
Abstract
Dyes are an essential food ingredient to improve sensory perception and draw consumers’ attention. This study aimed to intercalate dyes, Bordeaux Red (BR), Tartrazine Yellow (TY), and Bright Blue (BB) into layered zinc hydroxide salt by co-precipitation technique and to apply them in a cupcake food matrix. The intercalated dyes were characterized by X-Ray Diffraction (DRX), Fourier-Transform Infrared Spectroscopy (FTIR), Thermogravimetric analysis and differential Scanning Calorimetry. The cupcake color parameters L*, a*, and b* and its stability were evaluated for 15 days storage and compared to respective non-intercalated dyes. The BR and TY dyes intercalation was evidenced by DRX, while FTIR demonstrated characteristic bands of the non-intercalated dyes and the lamellar Hydroxysalt. Thermal analysis suggested an increasing degradation temperature for BR intercalated (LHS-BR). Red cupcakes elaborated with LHS-BR had lighter color characteristics evidenced by L* and a*. Yellow cupcakes elaborated with TY intercalated (LHS-TY), although had lighter color characteristics evidenced by L* and b* values, presented increased stability in comparison with TY during storage. New intercalated dyes application may be promising providing color stability gains for food matrices, improving products appearance and acceptance.
Keywords:Bakery; Bordeaux red; Bright blue; Layered zinc hydroxide salt; Tartrazine yellow
Introduction
Color is an essential sensory attribute to draw consumers’ attention [1], being used as additives that improve the presentation of a food. In a dye application procedure, beyond the coloring function, it is interesting to study the dye stability under real conditions of food production and storage, until it reaches the consumer’s table. Temperature and light exposition are considered both adverse factor for dye color and improve color stability mainly during storage is a relevant factor, regardless the processing technology used [2]. In order to improve color stability some intercalation procedures may be employed. As some organic anions do not have the sufficient thermodynamic driving forces to displace the intercalated nitrate anions from the layered zinc Hydroxinitrate structure, for example, new attempts can be made to produce hybrid compounds by direct synthesis. The direct synthesis to obtain layered compounds can be performed by various methods such as coprecipitation (or saltbase method) [3,4], Hydrothermal Synthesis [3]; and salt-oxide method [5]. Tons of dyes are used on a daily basis, mainly to improve or change food products’ color [6].
Some of artificial dye widely employed were Bordeaux red, Tartrazine yellow, and bright blue. Although these dyes are considered stables to heat and light, possibilities of gains in color and stability applied to foods are always interesting. Bakery goods are consumed worldwide with great acceptance among different public. Cupcakes are individual cakes portions of North American origin that conquered consumers of different age groups around the world. Traditionally cupcakes are produced with a baked dough that receives a topping. It is common to find cupcakes of different colors and flavors, which make them very attractive. In this< context, the possibility of colorful and/or stable dyes applied for cupcakes and for bakery sector are interesting [7]. In this study, the inorganic matrix layered zinc hydroxide salt was intercalated with Bordeaux red, tartrazine yellow, and bright blue by co-precipitation technique. The intercalated dyes were characterized and applied in cupcake formulation. The cupcake color parameters and stability were evaluated during storage and compared to respective nonintercalated dyes.
Materials and Methods
Materials e equipment’s
For this study, reagents of analytical grade were used. The dye Bordeaux red was supplied by Conditec (Medianeira, Brazil), and Tartrazine yellow and bright blue were supplied by Sensient (Milwaukee, USA). All reagents employed were analytical grade. The following equipment’s were employed: Magnetic stirring (Corning, 6796-420D, New York, USA), pHmeter (Even, PHS-3E), centrifuge (Cientec, CT 5000R, Belo Horizonte, Brazil), drying oven (New Lab, NL 80, Piracicaba, Brazil), X-Ray Diffraction (XRD) with a PANalytical diffractometer (Empyre, Malvern Panalytical, Westborough, USA), Fourier transform infrared spectrometer with an Attenuated Total Reflectance (ATR) (Spectrum 100s, Perkin Elmer, Beaconsfield, UK), Thermal-Analyzer (STA 6000, Perki Elmer, Beaconsfield, UK), blender (Power Max, Arno, São Paulo, Brazil), ultrasound bath (P60 H, Elmasonic, Singen, Germany), bakery oven (MPO 7948, Perfecta Curitiba, Curitiba, Brazil), and colorimeter (CR 410, Konica Minolta, Osaka, Japão).
Co-precipitation reaction to obtain hybrid dyes (LHSdye)
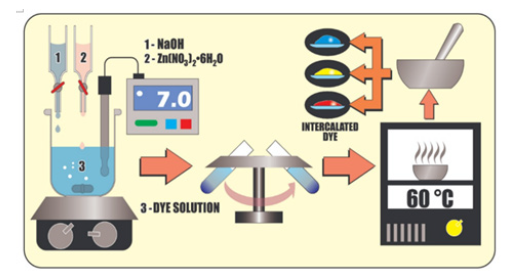
Dye-intercalated layered zinc hydroxide salts were synthesized by co-precipitation at alkaline pH. An aqueous solution of 1molL- 1 NaOH and 1.84, 4.53, and 1.40mol of Zn (NO3)2.6H2O aqueous solutions were slowly dropwise added to solution containing 0.0050, 0.0244, and 0.0030mol of Bordeaux red, Tartrazine yellow, and Bright blue dyes, respectively and final pH was adjusted to around 7. Based on the expected formula of the layered zinc hydroxide salt, Zn5(OH)8(Dye)2. nH2O, and to prevent any contamination, an excess of dye was used. The dispersion remained under magnetic stirring for 24h at room temperature (25 °C) and was subsequently rinsed with distilled water and centrifuged at 5000rpm for 7min at 25 °C. The process was repeated up to obtain colorless residual water. The solids obtained were dried in an oven-dried at 60 °C, macerated and stored up to characterizations and application (Figure 1).
Figure 1.Schematic representation of co-precipitation procedure to obtain hybrid dyes.
Samples characterization
The Zinc Hydroxynitrate (ZHN), Bordeaux Red (BR), Tartrazine Yellow (TY), Bright Blue (BB), Bordeaux Red Hybrid Dye (LHS-BR), Tartrazine Yellow Hybrid Dye (LHS-TY), and Bright Blue Hybrid Dye (LHS-BB) were characterized by X-Ray Diffraction (XRD) with CuKα=1.5418Å radiation source, 30mA current and 40kV voltage. Fourier-Transform Infrared Spectroscopy (FTIR) analyses were carried out with an Attenuated Total Reflectance (ATR) accessory with a zinc selenide crystal (ZnSe), 4 scans accumulation ranging from 600 to 4000cm-1 and 4cm-1 resolution. Thermogravimetric (TGA) and Differential Scanning Calorimetry (DSC) were carried out using a Thermal-Analyzer with a 20mLmin-1 oxygen flow rate and a 10 °C min-1 heating rate of up to 900 °C. The samples mass used ranged from 6 to 8mg.
Hybrid dye applied on cupcakes
The BR, LHS-BR, TY, and LHS-TY dyes were applied in cupcakes samples and color parameters were evaluated during shelf life. A cupcake base dough was produced using sugar (160g), wheat flour (160g), whole milk powder (20g), chemical yeast (15g), eggs (2ud), margarine (15g), and water (100mL). The BR (0.12g) and TY (0.12g) were directly added to cupcake base dough. Before LHSBR (0.80g) and LHS-TY (0.35g) addition to dough, the intercalation compounds were previously diluted on 100mL water and subjected to ultrasound treatment (37KHz frequency, 100% amplitude, 15min) to disaggregate and better disperse the intercalated pigment [1]. All ingredients were homogenized in a blender for 5min, arranged in paper molds nº 2 for cupcakes and baked at 150 °C for 15min. The samples were stored in a polyethylene bag at 22 °C for 15 days.
Color evaluation during storage
Cupcake samples were randomly selected for color analysis at predefined intervals of 5 days (0, 5, 10, and 15 days). The instrumental parameters of color luminosity (L*), green-red component (a*) and blue-yellow component (b*) were determined at ten different outer (top surface) and inner (after the cupcakes were cut in a latitudinal fashion just before reading) surface of the samples with the CIELAB system using a colorimeter calibrated with a D65 standard illuminant and a 10° angle. L* (lightness; 100=white, 0=black), a* (redness; +, red; −, green), and b* (yellowness; +, yellow; −, blue) values were obtained.
Statistical analysis
The results were presented as average±standard deviation, submitted to analysis of variance (one-way, ANOVA) followed by the Tukey test (p0.05) using the Statistica 7.0 software
Result and Discussion
Hybrid dye LHS-BR characterization
Figure 2.(A) X-ray diffractogram of (a) ZHN, (b) BR, and (c) e LHS-BR; (B) estimated size of BR dye molecule; (C) Structures of Lamellar Zinc Hydroxysalt intercalated with BR dye after sodium carboxylate coordination. (D) X-ray diffractogram of (a) ZHN, (b) TY, and (c) LHS-TY; (E) Estimated size of TY dye molecule; (F) Structures of Lamellar Zinc Hydroxysalt intercalated with TY dye after sodium carboxylate coordination. (G) X-ray diffractogram of (a) ZHN, (b) BB, and (c) LHS-BB.
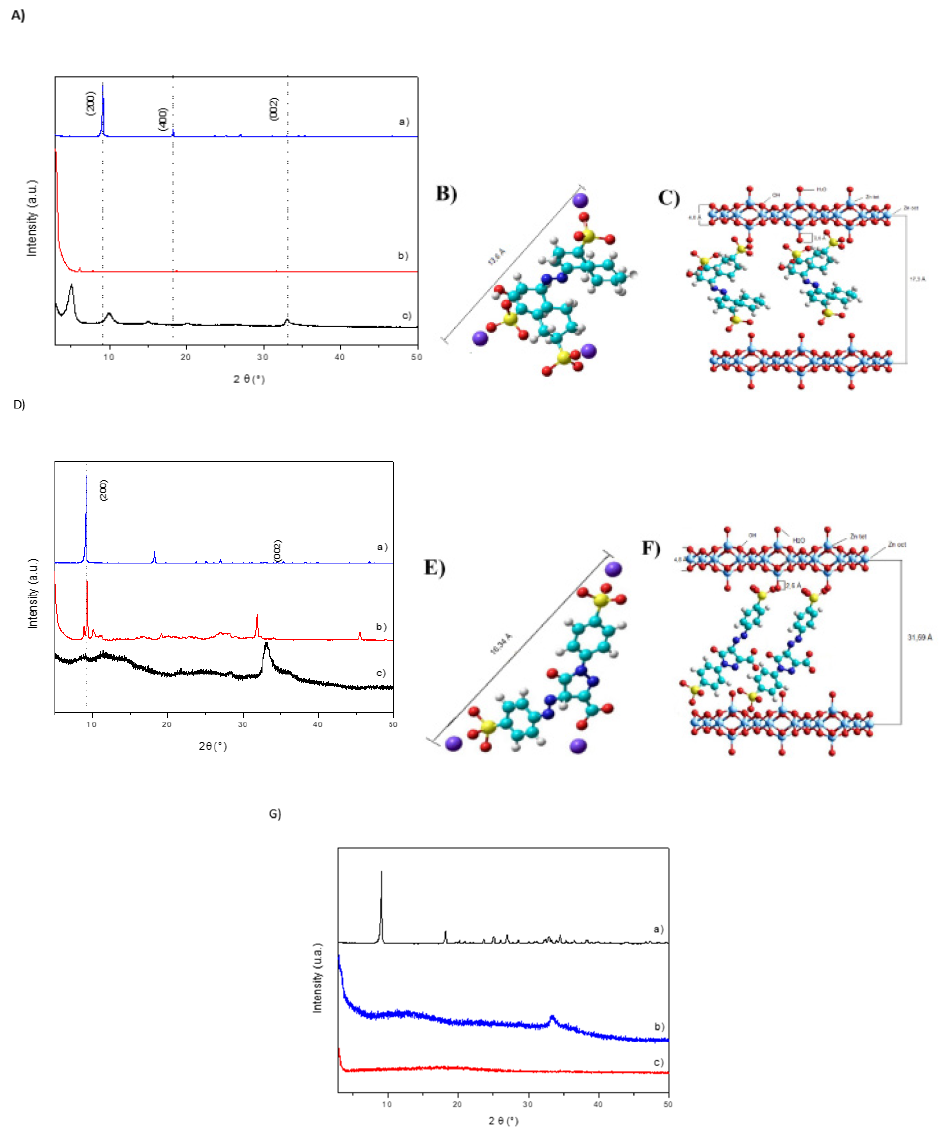
After the co-precipitation reaction, the obtained solid LHS-BR had similar color characteristic of non-intercalated dye. The LHS-BR L*, a*, and b* were 13.27±0.89, 15.52±0.22, and 8.96±0.09. The BR L*, a*, and b* were 12.64±0.19, 15.77±0.12, and 6.52±0.04. Analyzing the diffractogram (Figure 2a), the layered zinc Hydroxynitrate structure formation in the intercalation compound with BR may be observed by the peaks attributed to the basal reflection planes in the layered stacking direction (h00). Moreover, at 5 and 20° of 2*θ (degrees) regions the peaks 6.19 °C and 18.73 °C present a uniform distribution of the distances between them. Furthermore, a good crystallinity and lamellar repeat order and a basal distance of 17.6Å was evidenced. This distance is consistent with the size of the dye molecule calculated by the HyperChem program (13.6Å) (Figure 2b), allocated between the layered zinc Hydroxysalt (4.8 Å) also formed by two layers of tetrahedral zinc bonded to hydroxyl groups with each zinc tetrahedron an estimated size of 2.6Å. This characterizes the successful BB dye intercalation of a monolayer in the layered Hydroxysalt matrix as shown in Figure 2C [8].
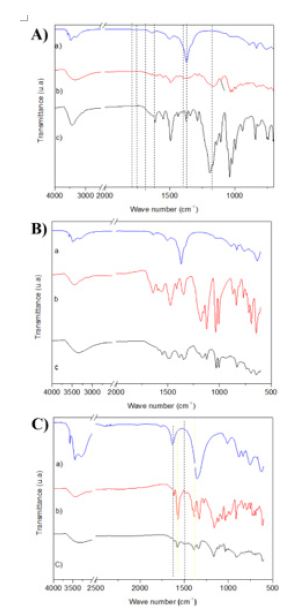
The FTIR spectra (Figure 3) corroborates the results obtained by the diffractograms, showing that the intercalation of the BR dye in layered Hydroxysalt was effective. At 1372cm-1 (LHS-BR) and 1370cm-1 (BR) a νs (S-O) was observer. At 1616 and 1492cm-1 (LHSBR) and 1588 and 1496 (BR) a links (N-N) ring was observed. At 1108 (LHS-BR) and 1106 (BR) a bonds (C-S) ring with the sulfonated groups (So3-) were observed. At 1620 (LHS-BR) and 1626 (BR) a C=C ring was observed. Note also two bands of the Carboxylate functional group (COO-), one at 1390cm-1 which corresponds to the symmetrical stretching and the second at 1552cm-1 referring to the asymmetrical stretching [9]. According to Nakamoto [10] the difference in frequency of the stretches (Δν=νas-νs), using as reference the Δν of the BR salt and the layered compound, indicates the form of coordination of the anion with the lamella metal. Therefore, monodentate coordination occurs when the Δν of the compound of interest is greater than the Δν of its salt. If the value of Δν of the compound of interest has approximate values, it indicates bridging between anion and the metallic center. When the Δν of the compound of interest is smaller than the Δν of the sodium salt, coordination will be bidentate [9,10].
Figure 3.(A) Vibrational spectra in the infrared region of (a) ZHN, (b) LHS-BR, and (c) and BR. (B) Vibrational spectra in the Infrared Region of (a) ZHN, (b) LHS-TY, and (c) and TY. (C) Vibrational spectra in the Infrared Region of (a) ZHN, (b) LHS-BR, and (c) and BB.
The frequency difference between the two bands gives
information about how the anion coordinates to the metallic center.
Thus, the delta of the sodium salt of the BR dye (Δν=152cm-1)
is smaller than the delta of the LHS/VB intercalated product
(Δν=160cm
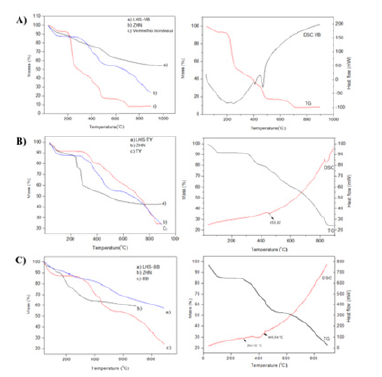
Figure 4.(A) Comparison between the thermal analysis of the (a) LHS-BR, (b) ZHN, and (c) BR. (B) Comparison between the thermal analysis of the (a) LHS-TY, (b) ZHN, and (c) TY. (C) Comparison between the thermal analysis of the (a) LHS-BB, (b) ZHN, and (c) BB.
Hybrid dye LHS-TY characterization
After the co-precipitation reaction, the solid obtained LHSTY showed a change in color. The LHS-TY L*, a*, and b* were 58.22±1.13, 33.66±0.50 and 79.43±1.65. The TY L*, a*, b* were 56.94±1.87, 43.97±0.30, and 76.18±2.11. LHS-TY had a lighter color (lower L*) and yellower color characteristic (grater b*). The X-ray diffractogram (Figure 2D) it is possible to verify that (ZHN) (Figure 2D) has a good lamellar repeat order crystallinity and an intense peak, referring to the plane (200), whose basal distance calculated by Bragg’s Law is approximately 9.8Å. For LHS-TY (Figure 2D) the formation of the layered structure of Zinc Hydroxynitrate was evidenced by the peak (002) at approximately 33 ºC, according to the file 24-1460 of the Joint Committee of Powder Diffraction Patterns (JCPDS) [8] and also presented well-defined diffraction peaks, lamellar repeat order and basal distance of 31.59Å. This distance is consistent with the presence of a monolayer of TY dye (16.34Å) (Figure 2C) in the lamellar structure formed by two layers of tetrahedral zincs linked to hydroxyls (4.8Å) with each Zinc Tetrahedron an estimated size of 2.6Å. This characterizes the success of the dye intercalation in the Lamellar Matrix as shown in Figure 2F [8].
The intercalation of the TY dye may also be confirmed by vibrational studies in the Infrared Region (FTIR) (Figure 3B), with characteristic bands for non-intercalated TY dye (Figure 3B). At 1028 and 1128 (LHS-TY) and 1124 and 1032 (TY) a s and as (SO3) were observed. At 1008 (LHS-TY) and 1004 (TY) (S=O) / (C6H5- SO2) ring was observed. At 3348 (LHS-TY) and 3448 (TY) a (O-H) of water and lamellae LHS were observed. At 648 and 692 (LHS-TY) and 644 and 716 (TY) a links (C-C), (C=C), and (C6H5) ring were observed. At 1548 (LHS-TY) and 1564 (TY) a (N=N) was observed. At 1340 and 1384 (HLS-TY) and 1348 and 1416 (TY) a (C-H) ring was observed. Morevoer, the bands at 1560cm-1 correspond to asymmetric stretching (νas) and bands at 1412cm-1 to Symmetrical Stretching (νs) of the COO-carboxylate group [14] Considering the symmetrical and asymmetrical stretching frequencies variation, the LHS-TY (164cm-1) and the dye salt (148cm-1), the intercalation product shows a monodentate coordination between the anion and the metallic center. Thermogravimetric analysis (Figure 4B) suggests mass losses between 0-100 °C for all samples, referring to the loss of absorption water. According to Leulescu et al. [15], the non-intercalated TY (Figure 4B) rsemains stable in the temperature range 110 °C to 200 °C, with a partial oxidation of the azo group (-N=N-) ocurring between 200 °C to 300 °C and with the decomposition initiated at 307.5 °C, correlated to an endothermic event at 453.02 °C, in which the loss of the carboxyl group and loss of the azo group occurs. The other mass losses observed may be related to the oxidation of SO3 and different aromatic groups. The LHS-TY (Figure 4B) had a mass loss profile similar to TY (Figure 4B), with degradation starting at 307.5 °C, while LHS-TY starts at 238.05 °C. As reported by Hongo et al. [16], several mass losses are observed, the main ones being related to the Dehydroxylation process of the lamellae and the nitrate present in the structure.
Based on the thermal analysis it was possible to estimate the amount of TY in the intercalation solid, which was considered the general lamellar formula of Zn5(OH)8(NO3)2-x(TY)x. nH2O, in which the percentage of solid without water of hydration and also the final percentage of zinc oxide were used. Assume that 5moles of zinc oxide (ZnO) are left for every mole of ZHN. Two main events were considered, the first referring to water loss (x1=104.35 and y1=92.076%) and the second referring to the amount of organic matter and Dehydroxylation of the lamellae lost in the burning of the material (ZnO) (x2=817.03 and y2=42.253%). Thus, it was possible to estimate the amount and the general formula of TY dye in the lamellar Hydroxysalt matrix, in which the sodium 2 carboxylate coordinates at two points in its structure (Figure 2F), therefore it proposes the composition of the LHS-TY compound with the formula Zn5(OH)8(NO3)1,28(TsY)0.72.3.72 H2O with molar mass 960.64g mol-1 [14]. Note that the initial degradation temperature of LHS-TY started at 242.11 °C, which is lower compared to the degradation temperature of the TY dye at 307.5°C.
Hybrid dye LHS-BB characterization
The diffractogram (Figure 2G) suggest that the BB intercalation (Figure 2G) did not show good crystallinity and neither did a basal distance value which corresponds to the intercalation of the dye in the layered matrix. However, as observed in the vibrational spectrum in the infrared region, (Figure 3C) the LHS-BB intercalation product (Figure 3C) shows characteristic bands of the BB blue dye (Figure 3C), referring to the stretching of the azo group (-N=N-) at 1500cm- 1, bands at 1620cm-1 attributed to the C=C stretching of the aromatic ring, and also symmetrical and asymmetrical stretching in the region of 1576cm-1 and 1392cm-1 from the COO- group [9,17]. The BB dye (Figure 4C) starts its thermal decomposition at 286.15 °C related to two endothermic events, one at 294.10 °C and another at 445.54 °C, referring to the combustion of organic matter. For the synthesized compound, it is noted that its decomposition started at 293.02 °C (Figure 4C). The analyses performed suggest that BB intercalation product was not successfully obtained.
Red cupcake color evaluation
L*, a*, and b* color parameters for red cupcakes are shown in Figures 5 & 6, and Table 1. The outer L* values range from 34.38 to 23.61 for BR, and from 39.78 to 28.94 for LHS-BR. The inner L* values range from 41.05 to 27.12 for BR, and from 44.91 to 33.32 for LHS-BR. The lower L* values represent a darker color. Up to 5 days, the red cupcakes kept its outer and inner surface L* value, while at 15 days a considerable outer and inner darker color was observed for both samples (p<0.05) (Figure 5A). The outer L* values were darker than inner ones, as expected due the crust color characteristic. Comparing the BR to LHS-BR cupcake samples, at all intervals the BR had a darker color. The outer a* values range from 43.70 to 34.93 for BR, and from 42.05 to 29.65 for LHS-BR. The inner a* values range from 44.58 to 33.24 for BR, and from 38.67 to 30.03 for LHS-BR. Higher positive a* values represent more intense red color. Up to 5 days, the red cupcakes kept its intense outer and inner red color evidenced by a* values, while at 15 days a significant outer and inner red color loss was observed for both samples (p<0.05) (Figure 5B). In general, the BR outer and inner red color was more intense than LHS-VB, due the higher a* values (p<0.05). The outer b* values range from 16.80 to 13.21 for BR, and from 15.56 to 13.95 for LHS-BR. The inner b* values range from 12.20 to 9.93 for BR, and from 10.48 to 9.58 for LHS-BR. Higher positive b* values represent more intense yellow color. During the 15 days, a significant outer and inner b* values reduction was observed, which was not observed for LHS-BR (Figure 5C). In contrast, at 15 days LHS-BR outer and inner b* values had a slight increase (p<0.05). In general, up to 15 days, the BR outer and inner yellow color was more intense than LHS-VB. However, at 15 days an inversion was observed, with higher b* values for LHS-BR compared to BR (p<0.05). An overall assessment of L*, a* and b* parameters for red cupcakes suggests that a comparable color stability was obtained for BR and LHS-BR. Mainly L* and a* values obtained for BR cupcakes reinforce the BR intercalation obtained employing ZHN, explained by the smaller color opening in the product using the same amount of dye, even using ultrasound in an attempt to facilitate the opening of the lamella. Regardless of the LHS-BR dye color opening applied to cupcake, the intercalated dye developed may provide stability gains for other food matrices, suggesting the importance of new applications.
Table 1:Red and yellow cupcakes color parameters of Luminosity (L*), green-red component (a*), and Blue-Yellow component (b*). BR: cupcake colored by Bordeaux Red; HSL-BR: cupcake colored by intercalated Bordeaux Red; TY: cupcake colored by tartrazine yellow; HSL-TY cupcake colored by intercalated Tartrazine Yellow. Mean±standard deviation (n=10) followed by different lowercase letters in the columns or different uppercase letters in the lines indicate a significant difference by Tukey’s test (p≤0.05).
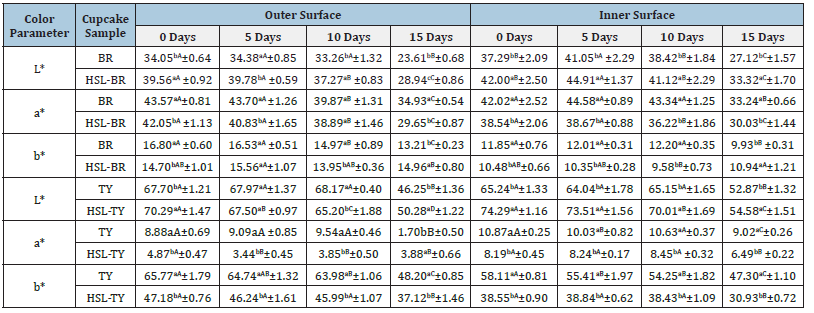
Figure 5.Red cupcake outer and inner color parameter of (A) Luminosity (L*), (B) Green-Red component (a*), and (C) Blue-Yellow component (b*). Yellow cupcake outer and inner color parameter of (D) luminosity, (E) Green-Red component, and (F) Blue-Yellow component.
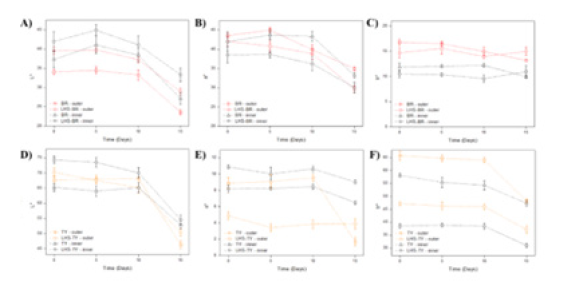
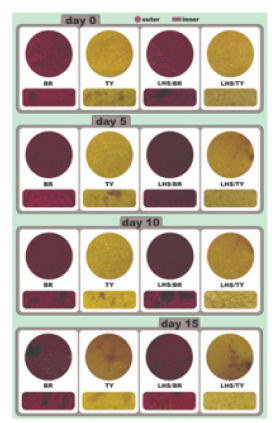
Figure 6.Color appearance of (A) Red and (B) Yellow cupcakes along 15 days of storage.
Yellow cupcake color evaluation
L*, a*, and b* color parameters for yellow cupcakes are shown in Figure 5 & 6, and Table 1. The outer L* values range from 68.17 to 46.25 for BR, and from 70.29 to 50.28 for LHS-BR. The inner L* values range from 65.24 to 52.87 for BR, and from 74.29 to 54.58 for LHS-BR. The lower L* values represent a darker color. Up to 10 days, the TY cupcakes kept its outer and inner surface L* value, while at 15 days a considerable outer and inner darker color was observed (p<0.05). For LHS-TY cupcakes, an outer and inner gradual darkening was observed during the 15 days of storage life (p<0.05). The outer L* values were darker than inner ones for, as expected due to the crust color characteristic. Comparing the TY to LHS-TY outer and inner cupcake samples color, TY had a darker color at initial and final storage time (p<0.05). Moreover, comparing outer and inner L* delta for TY and LHS-TY, the inner varied more between samples than outer ones (Figure 5D). The outer a* values range from 9.54.70 to 1.70 for BR, and from 4.87 to 3.44 for LHS-BR. The inner a* values range from 10.87 to 9.02 for BR, and from 8.45 to 6.49 for LHS-BR. Positive a* values represent the presence of red shades on samples. Up to 10 days, the yellow cupcakes kept its outer and inner red shades, while at 15 days a significant outer and inner red shade decrease was observed for both samples (p<0.05). The exception was the outer LHS-TY a* values, which decrease from 0 to 5 days and kept stable from 5 to 15 days (p>0.05).
The TY inner red shade was more intense than LHS-VB, due the higher a* values from 0 to 15 days (p<0.05). For outer a* values, the same was observed up to 10 days; at 15 days a marked decrease for TY a* value was observed in comparison with LHS-TY cupcake (p<0.05) (Figure 5E). The outer b* values range from 65.77 to 48.20 for BR, and from 47.18 to 37.12 for LHS-BR. The inner b* values range from 58.11 to 47.30 for BR, and from 38.84 to 30.93 for LHSBR. Higher positive b* values represent more intense yellow color. Although the more intense TY cupcakes yellow color, a significant outer and inner b* values reduction was observed for TY along the 15 days storage (Figure 5F & Table 1). For LHS-TY, the outer and inner yellow color obtained was kept up to 10 days (p>0.05), demonstrating LHS-TY dye greater stability in comparison with TY one. An overall assessment of L*, a* and b* parameters for yellow cupcakes suggests that TY cupcakes had more characteristic yellow color than LHS-TY. However, the decrease of b* values during storage observed for TY cupcakes reinforce TY color instability. The TY intercalation appears to have increased the stability of the dye applied to food matrix. Regardless of the LHS-TY dye color opening applied to cupcake, the intercalated dye provided stability gains for cupcakes and may provide stability gains useful for different food matrices, reinforcing the importance of new applications (Figure 6).
Conclusion
The intercalation of BR and TY dyes into layered zinc hydroxide salt was obtained and evidenced by X-ray diffractogram. The FTIR showed characteristic bands of the non-intercalated dyes and the layered Hydroxysalt. Thermal analysis suggested thermal stability with increasing degradation temperature for LHS-BR. Red cupcakes elaborated with LHS-BR had lighter color characteristics evidenced by L* and a* values. Yellow cupcakes elaborated with LHS-TY, although had lighter color characteristics evidenced by L* and b* values, presented increased stability in comparison with TY, especially observed from b* value during storage. The intercalated dyes developed may provide stability gains for other food matrices, suggesting the importance of new applications.
Acknowledgement
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES)- Finance Code 001, National Council for Scientific and Technological Development-Brazil (CNPq) (Process 315015/2018-7), and Araucária Foundation. The authors acknowledge Conditec Aditivos e Alimentos (Medianeira, Paraná, Brazil) and Sensient Technologies Corporation (Milwaukee, Wisconsin, USA) for the dyes supply. The authors acknowledge the CEANMED-Central Analytica Multiusuário of Universidade Tecnológica Federal do Paraná-Campus Medianeira, Paraná State, Brazil, by the assays performed.
Author Contribution
MAA carried out the experiments, elaborated figures and wrote the MS; DLK supervised the study, elaborated figures, and wrote the MS; NJP carried out the experiments, and edited the MS; PRSB carried out FTIR and Thermal Analysis, elaborated figures, and edited the manuscript; JCR edited the manuscript; RMG supervised the study, performed statistical analysis and edited the manuscript; CC carried out color analyses and edited the manuscript; ACTC conceived, supervised the study, carried out XDR analyses, and wrote the MS.
References
- Ongaratto GC, Oro G, Kalschne DL, Cursino ACT, Canan C (2021) Cochineal carmine adsorbed on layered zinc hydroxide salt applied on mortadella to improve color stability. Current Research in Food Science 4: 758-764.
- Arvanitoyannis IS, Stratakos AC (2012) Application of modified atmosphere packaging and active/smart technologies to red meat and poultry: A review. Food and Bioprocess Technology 5(5): 1423-1446.
- Reichle WT (1986) Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics 22(1): 135-141.
- Roy AD, Forano C, Malki KE, Besse JP (1992) Anionic clays: Trends in pillaring chemistry. In: Occelli ML, Robson HE (Eds.). Synthesis of microporous materials: Exapanded clays and other microporous solids 2: 108-169.
- Howe T, Lal M (1981) Studies of zinc-chromium hydroxy salts. I. thermal decomposition of [Zn2Cr(OH)6]X.nH2O, Where X-=F-, Cl- , Br-, I-, 12CO2-3, and NO-3. Journal of Solid State Chemistry 39(3): 368-376.
- Agócs A, Deli J (2011) Pigments in your food. Journal of Food Composition and Analysis 24(6): 757-759.
- Hopkin L, Broadbent H, Ahlborn GJ (2022) Influence of almond and coconut flours on ketogenic, gluten-free cupcakes. Food Chemistry: X 13: 100182.
- Cursino ACT, Gardolinski JEFDC, Wypych F (2010) Intercalation of anionic organic ultraviolet ray absorbers into layered Zinc Hydroxide Nitrate. Journal of Colloid and Interface Science 347(1): 49-55.
- Nakamoto K (1986) Infrared and raman spectra of inorganic and coordination compounds. Wiley, New York, USA, p. 496.
- Nakamoto K (2009) Infrared and raman spectra of inorganic and coordination compounds-Part B: Applications in coordination, organometallic, and bioinorganic chemistry. (6th edn), Wiley, New Jersey, USA, p. 400.
- Marangoni R, Bouhent M, Guého CT, Wypych F, Leroux F (2009) Zn2Al layered double hydroxides intercalated and adsorbed with anionic blue dyes: A physico-chemical characterization. Journal of Colloid and Interface Science 333(1): 120-127.
- Zimmermann A, Jaerger S, Wypych F, Zawadzki SF (2014) High-density polyethylene nanocomposites containing layered double hydroxides, intercalated with anions derived from azo dyes. Polímeros 24(3): 1-43.
- Silva MLN, Marangoni R, Cursino ACT, Schreiner WH, Wypych F (2012) Colorful and transparent poly(vinyl alcohol) composite films filled with layered zinc hydroxide salts, intercalated with anionic orange azo dyes (methyl orange and orange II). Materials Chemistry and Physics 134(1): 392-398.
- Cursino ACT, Zanotelli NC, Giona RM, Basso RLDO, Mezalira DZ (2021) Multifunctional food supplements based on layered zinc hydroxide salts intercalated with vitamin anions and adsolubilized with vanillin. Journal of Food Science and Technology 58(10): 3963-3971.
- Leulescu M, Rotaru A, Pălărie I, Moanţă A, Cioateră N, et al. (2018) Tartrazine: physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. Journal of Thermal Analysis and Calorimetry 134(1): 209-231.
- Hongo T, Iemura T, Satokawa S, Yamazaki A (2010) Chromate adsorption and pH buffering capacity of zinc hydroxy salts. Applied Clay Science 48(3): 455-459.
- Abadi FFSA (2021) Synthesis and characterization of azo dyes and study of the equilibrium and thermodynamics of adsorption of dyes on activated charcoal. Materials Today: Proceedings 49(7): 2699-2706.
© 2023 Kalschne DL. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






