- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Organic Curcuma Caesia Roxb. Extract Induces p21 Expression and G0/G1 Cell Cycle Arrest in FaDu Oropharyngeal Cancer Cells
Campos ADS1,2, Silva ACG3, Braga PAC4, Reyes FGR4, Fernandes PM5, Botelho PB6, Valadares MC3 and Horst MA1*
1Nutritional Genomics Research Group, Faculty of Nutrition, Federal University of Goiás – Goiânia, GO - Brazil
2Nutritional Epidemiology Observatory, Department of Social and Applied Nutrition, Institute of Nutrition Josué de Castro, Rio de Janeiro Federal University, RJ, Brazil
3Laboratory of Education and Research in In Vitro Toxicology, Faculty of Pharmacy, Universidade Federal de Goiás, Goiânia, GO, Brazil
4Department of Food Science and Nutrition, School of Food Engineering, University of Campinas – UNICAMP, Campinas, SP, Brazil
5Department of Phytosanitary, School of Agronomy, Federal University of Goiás – Goiânia, GO, Brazil
6Department of Nutrition, Faculty of Health Sciences, University of Brasília – Brasília, DF, Brazil
*Corresponding author:Maria Aderuza Horst, Faculdade de Nutrição - Universidade Federal de Goiás, Rua 227, Setor Leste Universitário, CEP: 74605-080 - Goiânia, GO - Brazil
Submission: October 04, 2022;Published: October 20, 2022

ISSN:2640-9208Volume6 Issue4
Abstract
The oropharyngeal cancer is the popular medicinal uses of Curcuma caesia Roxb. (C. caesia) include the treatment of cancer. We aimed to evaluate the cytotoxic potential of C. caesia extract in oropharyngeal cancer cells. Total phenolic compounds and secondary metabolites of C. caesia extracts were determined by the Folin-Ciocalteu and UPLC-QToF-MS methods, respectively. Cellular analyses were carried out in an oropharynx cancer cell line (FaDu) and in a control keratinocyte cell line (Hacat). The cytotoxicity assay was carried out by reduction of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Flow cytometry was conducted to evaluate cell cycle progression, and cell morphology was evaluated using Giemsa-May-Grunwald stain. Analysis of the intracellular Reactive Oxygen Species (ROS), Mitochondrial Membrane Potential (MMP), and the expression of p21, Bax and cytochrome c were conducted. The extract demonstrated dose-dependent cytotoxic activity in both cell lines. Increased retention was seen in the early stages of the cell cycle (G0/G1) in FaDu cells. Moreover, FaDu cells showed strong alterations in their morphology that suggested cellular apoptosis and there was increased expression of p21 in that line. The treatment with C. caesia extract shows no significant differences in ROS, cytochrome c or Bax in both cell lines. C. caesia extract reduces cell viability and induces apoptosis in FaDu cells without deleterious effects in Hacat cells. To our best knowledge, there has been no other study that described such effect in cancer cells due to the treatment of organic C. caesia extract.
Keywords: Cell viability; Oropharyngeal cancer; p21 expression; Apoptosis; Zingiberaceae; Phenolic compounds
Introduction
Published data indicate that nutraceuticals with antioxidant capacity can be useful as an adjuvant in anti-cancer treatment [1]. Cancer is one of the leading causes of death, and Head and Neck Squamous Cell Cancer (HNSCC) is one of the most common lethal malignancies worldwide [2]. They are responsible for 3 to 5% of all cancer’s cases registered worldwide [3] and the National Cancer Institute of Brazil (INCA) predicted that approximately 28,840 HNSSC cases to each year of the 2020-2022 biennium [4]. The multifactorial origin of HNSCC includes, in addition to genetic and epigenetic factors, environmental and lifestyle exposures such as tobacco and alcohol use, dietary imbalance, lack of physical activity and overweight and/or obesity, as well as reduced consumption of fruits and vegetables [5]. Once it is well-established that food consumption plays an important rule to protect against cancer due to their bioactive compounds, recently was demonstrated that the intake of minimally processed foods may reduce the risk of HNSCC [6], so their constituents (mainly vegetable secondary metabolites) may impact directly in carcinogenesis. Studies have been developed aiming to investigate its components and their mechanisms involved in the prevention and treatment of cancer [7]. Moreover, species belonging to the Zingiberaceae family have been described as having medicinal potential, including as the adjuvant agents [8], especially the Curcuma genus [9], among them Curcuma caesia Roxb. Curcuma caesia Roxb (C. caesia) is a perennial rhizome native to tropical and subtropical regions of Asia, Africa and Australia and has been widely produced in Thailand, Indonesia and Malaysia [10,11], as the tropical vegetable, C. caesia has been successfully cultivated in the central west region of Brazil. Also known as “black turmeric” or “Kala Haldi”, its medicinal uses include the treatment of inflammation, skin diseases, menstrual disorders, haemorrhoids, fever, vomiting, and asthma [12,13]. In addition, some studies have demonstrated antitumor activity in vitro against liver cancer cells HepG2 [14], Ehrlich ascites carcinoma in mice [13], as well in breast cancer cell line MDA-MB-231 [15]. The biological effects of the C. caesia rhizome can be attributed to its secondary metabolites, known as bioactive food compounds. Its secondary metabolite composition consists of camphor and phenolic compounds such as curcuminoids, alkaloids and others [12]. Eleven different terpenoids were also isolated from its rhizome; they had antitumor effect against breast, lung, colon, pancreas, and prostate cancers [11]. Among these components, furanodienone has already been described in the literature with antitumor activity [16]. Moreover, sesquiterpenoid compounds such as isocurcumenol have been described as having antitumor activity [17]. In the West, C. caesia is still not explored, and nothing is known about its therapeutic action and secondary metabolites in plants grown in Brazil, especially in plants cultivated in organic cropping system. Moreover, there are no data about the cytotoxic effect of its extract in cancer cells. Therefore, given the possibilities for antitumor effects, the main aim of this study was to evaluate the effects of C. caesia extract in oropharynx cancer cell line (FaDu).
Materials and Methods
Chemicals
Acetonitrile and HPLC grade methanol were purchased from J.T. Baker (J.T. Baker, Phillipsburg, NJ, USA). Folin-Ciocalteau phenol reagent and analytical grade formic acid were supplied by Sigma-Aldrich (St. Louis, MO, USA). Gallic acid (anhydrous) and Sodium carbonate (anhydrous) were purchased by Vetec® (Rio de Janeiro, RJ, Brazil). Ultrapure water was obtained from a Milli-Q purifier system, model Simplicity (Millipore, Bedford, MA, USA). Polytetrafluoroethylene (PTFE) filter membranes, 0.2μm porosity, used for the filtration of sample extracts for subsequent injection into the UPLC system, were purchased from Nova Analítica (São Paulo, SP, Brazil). Dulbecco’s Modified Eagle’s Medium (DMEM), Dimethyl Sulfoxide (DMSO) and Fetal Bovine Serum (FBS) were from Sigma-Aldrich (St. Louis, MO, USA). MTT [3-(4,5-Dimethyl- 2-thiazolyl)-2,5-tetrazolium bromide] was from Sigma-Aldrich (St. Louis, MO, USA). Giemsa-May-Grunwald stain was from Merck (Merck, Brazil). Leucine-enkephalin used as reference material for lock mass and 0.5mM sodium formate solution used for mass spectrometer calibration were supplied by Waters (Waters Milford, MA, USA).
Plant material
Organic rhizomes of C. caesia Roxb. were harvested in June 2017 from an organic farm located in Hidrolândia (16º57’48”south latitude and 49º11’2” west longitude), State of Goiás, Brazil. The plant was deposited in the herbarium of the Institute of Biological Sciences, Federal University of Goiás (registered number: 66.453). The rhizomes were washed, freeze-dried, and powdered. The powder was vacuum packed and then stored at -20 °C until analysis.
Proximate composition analysis and energy value estimation of the raw rhizome
The moisture content was determined by drying samples at 105 °C, and ash was obtained by incineration in an oven at 550 °C. The total dietary fibre and nitrogen content were determined according to AOAC [18] methods. The nitrogen content was analysed by using the micro-Kjeldahl method and converted to crude protein by using a conversion factor of 6.25. Total lipids were determined as described by Bligh & Dyer [19]. The digestible carbohydrate was estimated by difference, subtracting the values obtained for moisture, ash, protein, fibre and lipids. The energy value of the samples was determined by using the Atwater conversion factors of 4kcalg-1 for proteins and carbohydrates, and 9kcalg-1 for lipids [20]. All the analyses were performed in triplicate.
Extract preparation and total phenolic compound evaluation
The ethanolic extract was obtained according to the method reported by Gao et al. [21,22], with minor modifications. Briefly, 0.5g of C. caesia powder was dissolved in 15mL of 50% ethanol (1:1, v/v) and subjected to 30min in an ultrasonic bath (model 2800 A, Unique®, 40kHz, 154W, São Paulo, Brazil) at a frequency of 40kHz and a potency of 107W at room temperature. The mixture was centrifuged at 8,700g for 10min at 25 °C. The supernatant was filtered, the volume adjusted to 50mL with 50% ethanol. This extract was used to investigate Total Phenolic Contents (TPC). The total phenolic content was determined with Folin-Ciocalteu reagent according to Singleton & Rossi [23]. The total phenolic compound content was calculated from the calibration curve of gallic acid standard solutions and expressed as milligrams of Gallic Acid Equivalent (GAE)/g of dry weight by the absorbance measurement at 765nm, using the UV/Vis V-630 spectrophotometer (Jasco, Tokyo, Japan) [24]. The ethanolic extract was concentrated using a rotary evaporator under vacuum at 50 ºC and subsequently lyophilized. The dried extract was vacuum packed and kept in a freezer (-20 ºC) until application on cell lines.
UPLC-QToF-MS analysis of the secondary metabolites of C. caesia Roxb. Rhizome
The identification of secondary metabolites present in methanolic extract from C. caesia Roxb. rhizome was performed by UPLC-QToF-MS. The AcquityTM UPLC M-Class system coupled with a mass spectrometer model Xevo G2-XS QTof (Waters Milford, MA, USA) with electrospray source as ionization mode (ESI) were used. MassLynxTM software version 4.1 was used for data acquisition and data processing. For sample extraction, dried and ground rhizome of C. caesia was weighed (1g) into a 50-mL polypropylene tube, methanol (10mL) was added and the mixture was stirred in a vortex for 5min and then subjected to a further 5min in an ultrasonic bath. The mixture was centrifuged for 10min at 12,000g, at 20 °C, and the supernatant was transferred to an amber glass vial. The remaining precipitate was re-extracted twice to guarantee exhaustive extraction of the sample. The extracts were pooled in a single vial before injection into the UPLC-QToF-MS system. Chromatographic separation was carried out on an Acquity UPLC® BEH C18 column (1.7μm, 2.1mm×100mm) (Waters, Milford, MA, USA). A binary mobile phase composed of 0.1% formic acid solution (A) and acetonitrile +0.1% formic acid (B) was applied by using a gradient elution method as follows: 0min 95% A and 5% B; 10-11min 5% A and 95% B; 11.01-13min 95% A and 5% B. All analyses were performed at 30 °C with a flow rate of 0.35mL/min. The sample volume injected was 1μL, and all samples were filtered through PTFE membrane before injection into the LC system. Mass spectra were acquired in positive ionization mode using a mass range of m/z 100-1000. For the MS operating conditions, the following parameters were set: capillary voltage 3.0kV, sampling cone voltage 40V, source temperature 130 °C, desolvation temperature 600 °C, cone gas flow 50Lh-1 and desolvation gas flow 900Lh-1. Nitrogen was used as the desolvation gas, and argon was used in the collision cell. A solution of leucine-enkephalin at a concentration of 2ngg- 1 was used as reference solution (Lockspray) in positive mode in order to obtain the accurate mass of the target molecules, using the [M+H]+ ion at 556.2771 as the lock mass. For calibration of the equipment a 0.5mM sodium formate solution was infused at a flow rate of 10μL min-1.
Cell culture
The oropharyngeal cancer line (FaDu) was acquired by donation from the Laboratory of Molecular Biology of Cancer of the Federal University of São Paulo, UNIFESP (São Paulo, Brazil). The normal keratinocyte cell line (HaCat) was donated by the Laboratory of Education and Research in In vitro Toxicology (Tox In) of the Federal University of Goiás. The FaDu cells was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) and Nutrient Mixture F-12 Ham (1:1), supplemented with 10% (v/v) FBS (Sigma-Aldrich, St. Louis, MO, USA), and 1% Penicillin/Streptomycin (Gibco, Waltham, MA, USA). The HaCat cells were cultured in DMEM plus 1% Penicillin/Streptomycin and 10% FBS. Both cell lines were cultured in a humidified atmosphere 5% CO2 at 37 ºC.
Cytotoxicity assay
The effect of C. caesia dry extract was examined with the MTT assay. The cells were dispersed and seeded in a 96-well plate at a density of 1x104 cells/well. The plates were kept at 37 °C and 5% CO2 for 24h for cell adhesion. Subsequently, cells were treated for 24h with eight different concentrations of the C. caesia extract, ranging from 1.6 to 200μg of total Phenolic Compounds (PC)/ mLAfter exposure, 100μL of MTT (0.5mg/mL) solution prepared in medium was added to each well, and the cells were incubated at 37 °C and 5% CO2 for 3h. Immediately after, the supernatant was discarded, and MTT-formazan crystals were dissolved in 100μL of DMSO p.a. (Vetec, Rio de Janeiro, Brazil) under agitation for 20min at 30RPM on a plate shaker. The absorbance was read on a plate spectrophotometer (Multiskan Spectrum ThermoScientific®) at a wavelength of 560nm. Cell viability was determined by the comparison of absorbance from treated cells relative to the negative control (unexposed cells). Based on the MTT results, we established the concentration of extract that would produce 20% inhibition (IC20) of cell viability in FaDu cells, so that this concentration was employed in further mechanistic assays for both cell lines.
Cell morphology
To characterize cellular morphologic changes, the cells were stained with Giemsa-May-Grunwald. The cells (1x105 cells/well) were cultured on coverslips in 6-well plates and kept at 37 °C and 5% CO2, for 24h. Then, the cells were exposed to 14.2μg of PC/mL of extract and cultured for a further 24h. Subsequently, the medium was removed, and the cells were washed twice with 2mL of PBS at 37 °C and fixed in 1mL of 4% paraformaldehyde (pH 7.4) for 20min with shaking at 13RPM. Then, 1mL of Giemsa-May-Grunwald stain was added to each well for 30s. Immediately after, the wells were washed with deionized water to remove excess stain. The coverslips were dried under room temperature and transferred for microscopy slides containing 10μL of microscopy assembly medium. Cells were analysed under an optical microscope (DM 2000, Leica Microsystems, Bannockburn, USA).
Flow cytometric analysis
For flow cytometric assays, FaDu and HaCaT cell lines were cultured in 25cm³ culture flasks (5x105 cells/flask) for 24h for adhesion. Further, cells were exposed to 14.2μg (IC20) of PC/mL of extract for more 24h and harvested for the investigation of the parameters described in the following subsections. The analysis was performed in triplicate, and 10,000 events/tube were acquired in a flow cytometer (BD FACSCANTO II, BD Biosciences, NJ, USA).
Cell cycle progression: Cells were harvested individually and transferred to cytometer tubes, washed with PBS and centrifuged at 1500RPM. The cells were resuspended in 1mL of ice-cold 70% ethanol at 4–8 ºC for 24h. Then cells were washed with 2mL of cold PBS and resuspended in PBS containing 50μg/mL PI (Sigma- Aldrich, St. Louis, MO, USA) and 200μg/mL of RNase (Sigma- Aldrich, St. Louis, MO, USA).
Cytochrome c, Bax and p21 expression: Evaluation of the expression of the p21, cytochrome c and Bax proteins was performed with fluorochrome-conjugated monoclonal antibodies. cells were harvested, transferred to flow cytometer tubes and washed twice with 2mL PBS containing 0.1% of bovine serum albumin (BSA). Then, they were fixed with cytofix/cytoperm permeation fixation solution (BD Biosciences, NJ, USA) and incubated in a refrigerator at 4 °C for 20min. The cells were then washed twice with 2mL PBS-T20 (PBS plus 0.05% Tween 20). After washing, 50μL of each buffer solution (PBS-T20 and PBS-BSA) was added, and the antibody corresponding to the protein to be assayed was added to the tubes. The cells were incubated for more 30min at room temperature and in darkness. Immediately after, they were washed twice with 2mL PBS-T20 and suspended in 200μL PBS for flow cytometry analysis
Intracellular Reactive Oxygen Species (ROS) generation and mitochondrial membrane potential: To investigate ROS generation, the cells were washed three times with 2mL of PBSand then suspended in 300μL of 10μM Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) for ROS measurement. The tubes containing this mixture were incubated at 37 °C for 1h and analysed by flow cytometry. For mitochondrial membrane potential and the cells were washed with 2mL of PBS and further incubated at 37 °C and 5% CO2 for 1h with 1μg/mL Rhodamine-123 dye solution. Immediately after, they were washed again and analysed by flow cytometry.
Clonogenic assay
To carry out the clonogenic assay, the cells were removed from the culture flasks, transferred to 6-well plates (1000 cells/well) and incubated at 37 °C and 5% CO2, for 24h. After adherence, the cells were treated with decreasing concentrations of the extract (14.2, 7.1 and 3.5μgCF/mL) for 24h, so that the cells were washed with 2mL of PBS and cultured regularly for another 7 consecutive days. The cells were then washed twice with 2mL of PBS and fixed with 1mL of methanol (Vetec, Rio de Janeiro, Brazil) for 2min. Immediately after, the cells were exposed to 1mL of Giemsa-May- Grunwald stain for 10min and washed with deionized water. After complete drying, the number of colonies was analyzed, and the plates were photographed (BioSpectrum® Imaging System, UVP, Upland, Canada). The areas on the plates occupied by colonies were quantified by using ImageJ software (NIH, USA).
Statistical analysis
The results were presented as mean±SEM. Statistical analyses were performed using GraphPad Prism 5 (GraphPad® Software CA). The determination of Inhibitory Concentrations of 50% (IC50) and 20% (IC20) cell viability was performed through non-linear regression. The Student’s t-test was used to compare continuous variables between the control and treated groups and the one-way ANOVA followed by Tukey’s post hoc test, to compare more than two treatments P values of<0.05 were considered statistically significant.
Results and Discussion
Proximate composition
Use of medicinal plants is probable the oldest method to treat illness in the human history and now these plants have attracted attention for this phytotherapeutic potential. Among these plants we have Curcuma caesia Roxb. been used for centuries in traditional medicine in many Asian countries. However, little is known about its centesimal composition and secondary metabolite content, as well its cytotoxic potential in oropharyngeal cancer cells. Moreover, to our knowledge, there is no data available about this rhizome cultivated in organic cropping systems. The identification of compounds with biological activity and its therapeutic effect in this type of cancer could support future research and use of this rhizome as a source of compounds to be isolated and applied in clinical routine. The macronutrient composition characterizes the food nutritional quality and may vary depending on the growing region and climatic conditions. Nutritional characterization of the C. caesia rhizome is shown in Table 1. The rhizome has a high moisture content (82.14%), as reported in studies with another species belonging to the same genus [25,26]. Moreover, fiber (2.71g/100g), lipid (1.85g/100g), and digestible carbohydrate (11.7g/100g) were higher in contents, when compared with similar species [25]. However, ash (0.82g/100g) and protein contents were lower [25]. This disparity in the results can be explained by different factors, such as geographic region and cropping system, harvesting period, mode of sample processing or genetic variation [27].
Table 1:Proximal composition of Curcuma caesia rhizome (g/100g) of fresh sample.
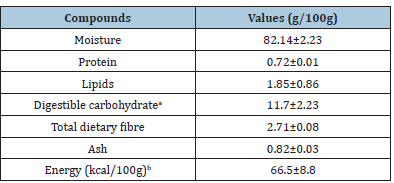
Values are expressed in mean+standard deviation. aTotal carbohydrate was estimated by difference. bEnergy value was calculated considering the Atwater conversion factors of 4 for protein and carbohydrate, and 9 for lipid.
Phenolic compounds in C. caesia Roxb. and identification of secondary metabolites,
Studies have shown that the use of a partially polar solvent has a higher extraction capacity of antioxidant compounds, such as phenolic compounds [28,29]. The C. caesia Roxb. rhizome content of total phenolic contents was 4.5mg AGE/g of dry sample. This result is higher than reported by Devi et al. [30] and comparable to the one obtained for C. zedoaria rhizome (5.9mg AGE/g dry sample) [31]. Therefore, it can be inferred that this difference could be a result of the type of solvent used for extract preparation. Methanol, for example, has been described as a better solvent to extraction of the TPC [32,33]. Another possible explanation is that the sample used in the present study is an organic crop, which may stimulate the synthesis of Phenolic Compounds (PC) responsible for the plants’ natural defences [27]. These PC are secondary plants’ metabolites and their levels in plants may vary widely by influence of growing conditions, moisture, and attack by plant pathogens [34]. Furthermore, there is an increased interest in health benefits of these compounds to humans. Researchers have demonstrated that increased consumption of fruits and vegetables, which increase the PC intake, decrease the risk of cancer [35] and cardiovascular disease [36]. Thus, the identification of these compounds in plants traditionally used to medicine is very important to support its use. The analysis of mass spectra obtained shows that the extract of C. caesia Roxb. has terpene compounds as mainly secondary metabolites, more specifically sesquiterpenes, the structures of which are shown in Figure 1. Only two phenolic compounds are suggested: tetrahydro curcumin, a curcuminoid type, and turmeronol, a phenolic sesquiterpene. From the accurate mass obtained in the low-energy collision spectra, it was possible to determine the elemental composition of the molecules with a high probability of certainty. Thus, from the molecular formula, it was possible to deduce some chemical structures for the compounds. Through the database KNApSAcK (http://kanaya.naist.jp/ KNApSAcK/), it was possible to survey the presence of compounds with the molecular formulas proposed by the elemental composition tool in the Curcuma genus, more specifically in C. caesia. The results are in agreement with data reported in the literature for the genus Curcuma, as well as for the species C. caesia Roxb [37].
Figure 1.Proposed structures of compounds found in methanolic extract from the C. caesia rhizome.
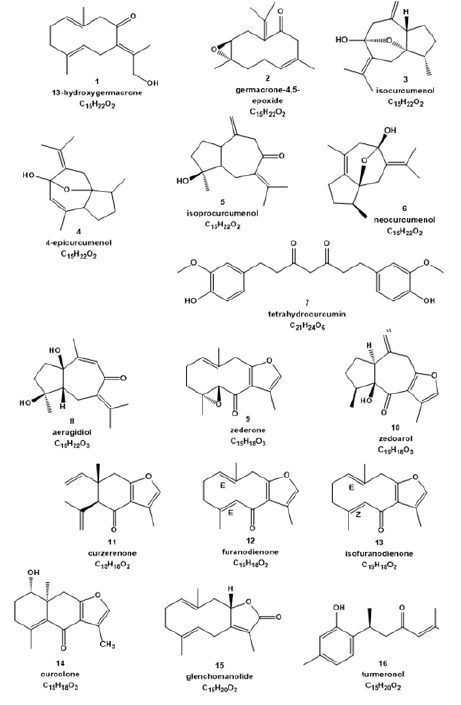
Analysing the high-energy collision mass spectra, it was possible to observe the fragmentation profile of the molecules present in the extract and, therefore, to deduce the possible losses of mass/charge. In some cases, the molecular formula presents several isomers that show a very similar fragmentation profile, which does not allow us to unambiguously conclude which isomer is involved. However, based on the molecules reported in the literature for this species, we can propose the structures described in Figure 1. Table 2 shows the molecular formulas determined by the Elemental Composition tool, the accurate mass (as well as the mass error determined), the fragmentation profile and the possible structures for each substance observed in the extract. The references cited in Supplementary Table 2 present a survey of the compounds previously described in the literature for species of the Curcuma genus, which afforded 13-hydroxygermacrone [1], germacrone-4,5-epoxide [2], isocurcumenol [3], 4-epicurcumenol [4], isoprocurcumenol [5], neocurcumenol [6], tetrahydrocurcumin [7], aerugidiol [8], zederone [9], zedoarol [10], curzerenone [11], furadienone [12], isofuranodienone [13], curcolone [14], glenchomanolide [15], and turmeronol [16]. Many of these compounds have been described in the literature as having antitumor activity against several cancer cells lines, such as isocurcumenol [17], furanodienone, aerugidiol [16], tetrahydrocurcumin [37,38], and curcuzederone [15].
Table 2:Molecular formula, accurate mass, and main fragments found for the secondary metabolites present in the methanolic extract from of C. caesia rhizome. *RT: Retention time

Cytotoxicity evaluation by inhibition of cell viability and cell cycle progression
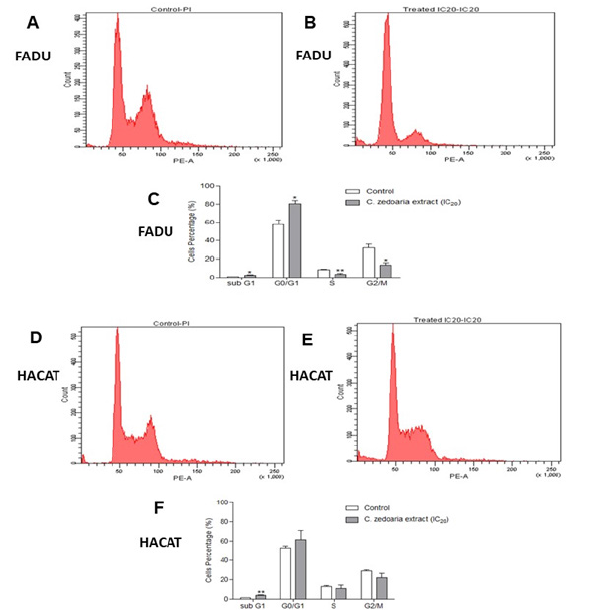
Carcinogenesis is a multistep process and has traditionally been separated in three different phases: initiation, promotion, and progression [39,40]. The first one, is characterized by being rapid and involves irreversible DNA damage that may cause mutations. Such mutations deregulate the signalling of biochemical pathways associated with cell proliferation and differentiation. Promotion phase is the longest and is a consequence of the functional loss of regulatory proteins and cellular checkpoints that play rules in proliferation and apoptosis [40,41]. In the final phase (progression), there is a fast increase in the tumor size and cellular changes that characterize the cancer itself with classic alterations such as: selfsufficiency in growth signalling, insensitivity to growth-inhibitory signals, inhibition of apoptosis, limitless replicative potential, and capacity for angiogenesis [39]. In addition, promotion stage is considered reversible, also is the point at which natural or synthetic chemical agents can reduce cell proliferation [40,42,43]. Thus, we investigated the potential that the bioactive compounds of the ethanolic extract of C. caesia could have in human’s tumor cells. To evaluate the effect of C. caesia on the reduction of cell viability, Fadu and HaCat cells were treated with eight concentrations ranging from 200 to 1.6μg TE PC/mL for 24h. The dried C. caesia extract showed a concentration-dependent inhibitory effect on cell growth regarding FaDu and HaCat cells. The IC50 value was 30.13μg TE PC/ mL in FaDu and 13.36μg TE PC/mL in HaCat. These results show that C. caesia extract has the potential to inhibit cell proliferation in both lines. This inhibition may be induced by the presence of compounds such as isocurcumenol and tetrahydrocurcumin, characterized as able to inhibit the proliferation of tumor cells [17,38]. Furthermore, the cell cycle progression was also altered by treatment. As shown in Figure 2; C. caesia extract induced retention in the first phase of the FaDu cell cycle. The proportion of G0/G1 phase cells was increased from 57.2% to 80.4% in 24h of treatment. Nevertheless, the HaCat line showed a slightly increased number of cells in the Sub-G1 phase. Thus, we can highlight that the extract plays an important role in the cell cycle of neoplastic cells, retaining cells in the first checkpoint. Moreover, this effect is not observed with the same proportion in HaCat cells.
Figure 2.Cell cycle phases of FaDu and HaCat cells. The histograms show untreated FaDu cells (A) and those treated for 24h (B) with C. caesia extract at a concentration of 14.2μg TE of phenolic compounds/mL (IC20). Figure (C) represents the percentage of FaDu cells treated and not treated with the C. caesia extract in the different phases of the cell cycle. *p<0.05 and **p<0.01. The histograms show untreated Hacat cells (D) and those treated for 24h (E) with extract at a concentration of 14.2μg TE of phenolic compounds/ mL. Figure (F) represents the percentage of Hacat cells treated and not treated with the extract of C. caesia in the different phases of the cell cycle. Measurements were performed on three independent experiments (**p<0.01).
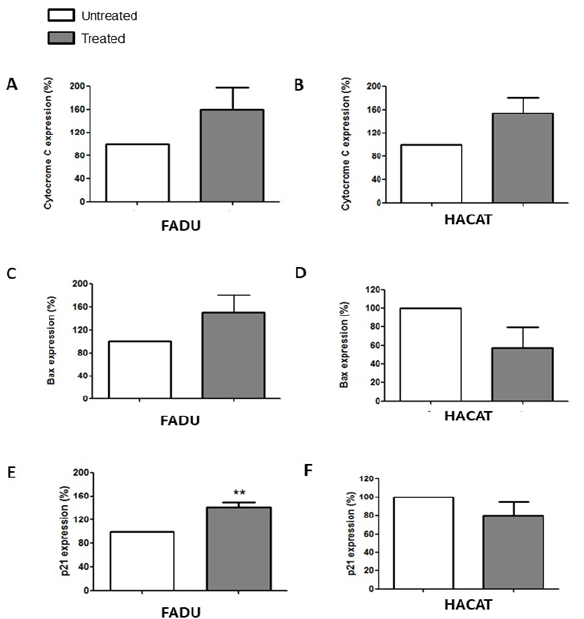
Morphologic changes and cytochrome c, Bax and p21 expression
Morphological analysis of FaDu cells exposed for 24h to C. caesia extract at the concentration corresponding to its IC20 revealed morphological changes suggesting cell death by apoptosis. Among these alterations were marked cytoplasmic vacuolization, chromatin condensation, cell degeneration, cellular pleomorphism and nuclear fragmentation (Figure 3). Regarding Hacat cells, no marked changes were observed in the overall morphology of the cell, only a slight increase in vacuolization in the treated cells compared with the untreated cells (Figure 3). These findings reinforce the hypothesis that C. caesia induces cell death by apoptosis in tumor cells. Dysregulation of apoptosis frequently occurs in cancer, and cancer cells escape apoptosis through different mechanisms. Apoptosis is associated with morphological changes such as chromatin condensation, nuclear fragmentation and cytoplasmic shrinkage. After the induction of apoptosis, other morphological changes occur, like loss of membrane integrity, structural changes in organelles and the formation of apoptotic bodies [44,45]. Such classical morphological signs were observed in FaDu cells treated with C. caesia extract, suggesting that it can induce apoptosis. Nevertheless, these signals were not observed in the Hacat cell line. Assessment of the expression levels of cytochrome c, Bax and p21, proteins connected directly to the apoptotic process, showed that only p21 was affected by treatment with the extract of C. caesia and only in FaDu cells (Figure 4). Such effect suggests that C. caesia demonstrated some grade of tumor cells-specificity regarding the modulation of this signaling pathway. Cell cycle progression is characterized by a sequence of events that allows cells to duplicate their genetic material and form two daughter cells with equal DNA content [58]. The cycle has checkpoints between its phases in order to correct possible mistakes in the genetic material, the first checkpoint being between the G1/S phases. Thus, when DNA damage is detected in the G1 phase, the cell cycle is stopped, retaining cells at this stage to repair the genetic material or induce cell death by activating the expression of some genes, such as p21 [46]. The p21 protein has several functions, including cell cycle arrest in the G1 phase, transcriptional regulation, apoptosis and induction of DNA repair. The literature suggests that the p21 protein performs tumour suppressor activity independently of the p53 protein [47,48]. Based on the importance of apoptosis lose control in carcinogenesis, many studies have targeted the pro-apoptotic protein p21 to develop new drugs for the cancer treatment. Anticancer agents, including histone deactylase (HDAC) inhibitors, exhibit anticancer efficacy through their ability to promote p21 induction [49]. The higher p21 expression can increase apoptotic susceptibility to anticancer drug cisplatin in human ovarian [50] and in hepatoma cells [51]. These findings raise the potential that natural agents that increase p21 expression may be useful to reduce cisplatin doses and reduce treatment toxicity, and still obtain similar responses at higher doses. Our results show the corroborating cell cycle arrest in the G1 phase and a significant increase in the expression of p21, indicating that the organic C. caesia extract likely induces apoptosis through the activation of p21. To our knowledge, to date, there has been no other study that described such effect in cancer cells due to the treatment of C. caesia extract.
Figure 3.Morphological changes caused by exposure of FaDu and HaCat cells to extract of C. caesia (14.2 μg/mL TE of phenolic compounds) for 24h and stained with Giemsa. (A) Untreated FaDu cells had Dispersed Chromatin Nuclei (DCN). (B) FaDu cells treated with extract showed Cytoplasmic Vacuolization (CV), Chromatin Condensation (CC), Cell Pleomorphism (CP), Nuclear Fragmentation (NF) and Cell Degeneration (CD). (C) Untreated HaCat cells had Dispersed Chromatin Nuclei (DCN). (D) HaCat cells treated with extract showed only discrete Cytoplasmic Vacuolization (CV).
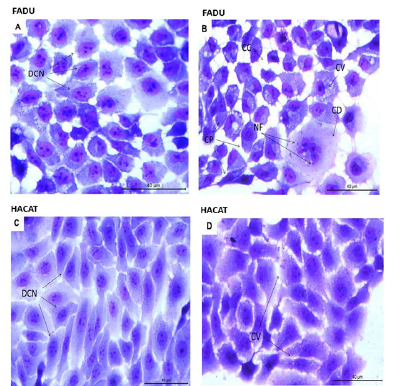
Figure 4.Expression of cytochrome c, Bax and p21 proteins in the FaDu and HaCat lines after 24h of exposure to 14.2μg TE phenolic compounds/mL of C. caesia extract. Histograms (A, C and E) show the fluorescence intensity expressed by the proteins analysed in the absence and presence of the extract in FaDu cells. The histograms (B, D and F) show the fluorescence intensity in the HaCat line treated and untreated with the extract at the same concentration and for the same period. Each bar presents the mean ± SD of three independent experiments (**p<0.01).
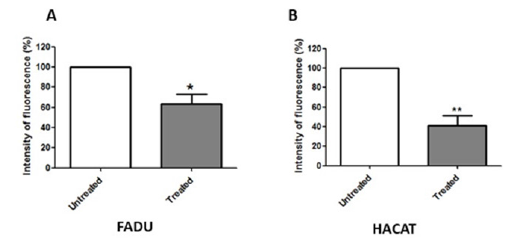
Mitochondrial membrane potential and ROS production
Mukunthan et al. [14] exposured human liver adenocarcinoma (HepG2) cells to hexane rhizome extract of C. caesia, the authors observed apoptosis with decreased expression of antiapoptotic proteins and increased expression of key regulators of mitochondrial apoptotic pathways. Besides mitochondrial apoptoses, membrane potential is considered an important indicator of mitochondrial status, as well as a feature commonly altered in different cell death mechanisms [52]. In our evaluation, both cell lines exposed to the C. caesia extract had a significant decrease in mitochondrial membrane potential (Figure 5). This indicates that tumour cell recruitment of the intrinsic pathway may have occurred during the cell death process. The intrinsic apoptosis pathway, also called the mitochondrial pathway, is activated in response to internal cell stress, which can be caused, for example, by DNA damage. In the activation of intrinsic apoptosis pathway, pro-apoptotic proteins can act on the mitochondrial membrane, increasing its permeability and, consequently, releasing proteins present in its intermembrane space, such as cytochrome c, into the cytoplasm [53]. These proteins, in turn, activate the caspase cascade, which will culminate in cell death [54,55]. This ability is an important effect of compounds with potential as antitumor therapies.
Figure 5.Mitochondrial membrane potential of FaDu (A) and HaCat (B) lines, after 24h of exposure to the extract. The graphics show the fluorescence intensity of treated cells relative to that of untreated cells. The bars represent the mean±SD of three independent experiments (*p<0.05 and **p<0.01).
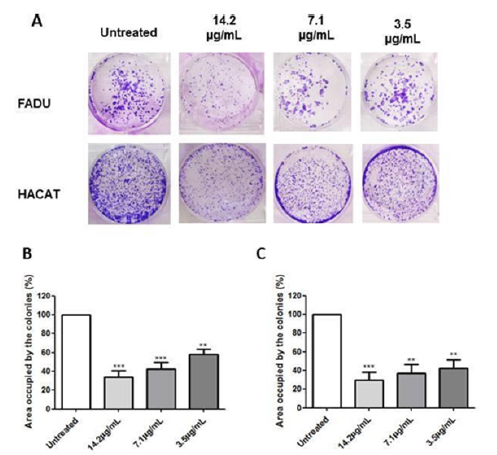
Effect of C. caesia extract on colony formation
The clonogenic assay can detect cells that remain reproducible, even after receiving treatments that damage the cell [56]. The results of our study demonstrated that the extract of C. caesia promotes significant inhibition of cell growth in both lines (Figure 6). In the tumor cell line, the highest inhibition rate was obtained with a concentration of 14.2μg TE PC/mL. However, we observed increasing reversibility as the applied concentration decreased. On the other hand, HaCat cells demonstrated greater reversibility in all tested concentrations, which might indicate that the extract has selective activity against cancer cells. Also, this finding may be related to the fact that there was no upregulation of the expression of the p21 protein in the basal keratinocytes, which is related to the greater reversion of cell growth arrest [57].
Figure 6.Clonogenic assay performed with the FaDu and HaCat cell lines to evaluate the potential for reversible action of the C. caesia extract. (A) Wells containing the colonies of both lines after 24h of exposure to the extract at concentrations of 14.2, 7.1 and 3.5μg TE phenolic compounds/mL. Soon after, the treatment was suspended, and the cells were cultured for another 7 days. Graphical representation of the area occupied by the colonies formed in the FaDu (B) and HaCat (C) lines referring to each concentration of the extract.
Conclusion
Taken all together, these findings suggest that the C. caesia extract can decrease cancer cell viability with morphological changes and induce protein expression that indicates apoptosis in the FaDu cell line [58-65]. The extract plays roles against the oropharyngeal cancer cell line, arresting the cells at the first checkpoint of the cell cycle, increasing p21 expression, decreasing the mitochondrial membrane potential and causing changes in the cell’s morphology that indicate apoptosis [66-70]. In contrast, these effects were not pronounced in the HaCat cell line. Further studies in vitro and in vivo are necessary to further investigate the biologic effects of C. caesia extract on cancer patients. Thus, in the future, this rhizome may be useful to prevent, treat or as adjuvant in oropharyngeal cancer by oral consumption or even as source to isolate compounds with biological effects.
Acknowledgement
The scholarship from the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior“(CAPES), Brazil. In addition, we would like to thank Andre Luiz Vettore and Viviane Carlin for donation of the FaDu cells. The authors would like to thank Waters Brazil (São Paulo, SP, Brazil) for supplying the UPLCXevo G2-XS QToF system employed in this study..
Ethics Statement
This research does not include any human or creature testing..
References
- Villegas C, Perez R, González-Chavarría I, Paz C (2021) Curcuma as an adjuvant in colorectal cancer treament. Life Sciences 286: 120043.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Cho J, Johnson DE, Grandis JR (2017) Therapeutic implications of the genetic landscape of head and neck cancer.
Semin Radiat Oncol 28(1): 2-11. - Brazil (2019) José Alencar Gomes da Silva National Cancer Institute. 2020 estimate: cancer incidence in Brazil. INCA.
- Winkels R.M, Van Lee L, Beijer S, Bours MJ, Van Duijnhoven FJB (2016) Adherence to the world cancer research fund/american institute for cancer research lifestyle recommendations in colorectal cancer survivors. Cancer Medicine 5(9): 2587-2595.
- Podestá OPG, Peres SV, Salaroli LB, Cattafesta M, Podestá JRV, et al. (2019) Consumption of minimally processed foods as protective factors in the genesis of squamous cell carcinoma of the head and neck in Brazil. Plos One 14: 1-19.
- Arora I, Sharma M, Tollefsbol TO (2019) Combinatorial epigenetics impact of polyphenols and phytochemicals in cancer prevention and therapy. Int J Mol Sci 20(18): 4567.
- Chen M, May BH, Zhou IW, Xue CCL, Zhang AL (2016) Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: Contributions of specific plants to tumor response.
Integr Cancer Ther 15(1): 40-59. - Awin T, Mediani A, Maulidiani, Shaari K, Faudzi SMM, et al. (2016) Phytochemical profiles and biological activities of Curcuma species subjected to different drying methods and solvent systems: NMR-based metabolomics approach. Industrial Crops and Products 94: 342-352.
- Asem SD, Laitonjam WS (2012) Investigation of the structure-nonlinearity relationship of zederone from the rhizomes of Curcuma caesia Indian Journal of Chemistry 51B: 1738-1742.
- Liu Y, Roy S, Nebie RHC, Zhang Y, Nair MG (2013) Functional food quality of Curcuma caesia, Curcuma zedoaria and Curcuma aeruginosa endemic to northeastern India. Plant Foods Hum Nutri 68(1): 72-77.
- Devi HP, Mazumder PB (2016) Methanolic extract of Curcuma caesia Roxb. prevents the toxicity caused by cyclophophamide to bone marrow cells, liver and kidney of mice. Pharmacognosy Res 8(1): 43-49.
- Karmakar I, Dolai N, Kumar RBS, Kar B, Roy SN, et al. (2013) Antitumor activity and antioxidant property of Curcuma caesia against Ehlich’s ascites carcinoma bearing mice. Pharm Biol 51(6): 753-759.
- Mukunthan KS, Satyan RS, Patel TN (2017) Pharmacological evaluation of phytochemicals from South Indian black tumeric (Curcuma caesia) to target Cancer apoptosis. J Ethnopharmacology 209: 82-90.
- Al-Amin M, Eltayeb NM, Khairuddean M, Salhimi SM (2019) Bioactive chemical constituents from Curcuma caesia rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Natural Product Research 35(18): 3166-3170.
- Zhao Y, Wang C, Chow AHL, Ren K, Gong T, et al. (2010) Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of zedoary essential oil: Formulation and bioavailability studies. Int J Pharm 383(1-2): 170-177.
- Lakshmi S, Padmaja G, Remani P (2011) Antitumour effects of isocurcumenol isolated from Curcuma zedoaria rhizomes on Human and Murine cancer cells. Int J Med Chem.
- AOAC-Association of Official Analytical Chemists (2010) Official methods of analysis of the Association of Official Analytical Chemists. AOAC: Washington DC, USA.
- Bligh EG, Dyer WL (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8): 911-917.
- Merril AL, Watt BK (1973) Energy value of foods: basis and derivation. Agriculture Handbook, Washington DC, Department of Agriculture, ARS, United States, 74.
- Gao XF, Li QL, Li HL, Zhang HY, Su JY, et al. (2014) Extracts from Curcuma zedoaria inhibit proliferation of human breast cancer cell MDA-MB-231 In Vitro. Evid Based Complement Alternat Med 1: 1-9.
- Shirsath SR, Sable SS, Gaikwad SG, Sonawane SH, Saini DR, et al. (2017) Intensification of extraction of curcumin from Curcuma Amada using ultrasound assisted approach: Effect of different operating parameters. Ultrasonic Sonochemistry 38: 437-445.
- Singleton VL, Rossi JJA (1965) Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid regent. American Journal of Enology and Viticulture 16: 144-158.
- Santiago GL, Oliveira IG, Horst MA, Naves MMV, Silva MR (2018) Peel and pulp of Baru (Dipteryx alata) provide high fiber, phenolic content and antioxidant capacity. Food Sci Technol 38(2).
- Leonel M, Sarmento SBS, Cereda MP (2003) New starches for the food industry: Curcuma longa and Curcuma zedoaria. Carbohydrate Polymer 54(3): 385-388.
- Yue PF, Lu XY, Liao MX, Yuan HL, Zhu WF (2010) The effect of oil components and homogenization conditions on the physicochemical properties of zedoary turmeric oil submicron emulsions. J Disper Sci Technol 31(11):1535-1540.
- Smith-Spangler C, Brandeau ML, Hunter GE, Bavinger JC, Pearson M, et al. (2012) Are organic foods safer or healthier than conventional alternatives? A systematic review. Ann International Med 157(5): 348-366.
- Giada ML (2014) An approach to the in vitro antioxidant capacity of plant foods and beverages. Demeter 9: 137-146.
- Reenu J, Azeez S, Bhageerathy C (2015) In vitro antioxidant potential in sequential extracts of curcuma caesia rhizomes. Indian J Pharm Sci 77(1): 41-48.
- Devi HP, Mazumder PB, Devi LP (2015) Antioxidant and antimutagenic activity of Curcuma caesia Roxb. Toxicol Rep 14(2): 423-428.
- Tariq S, Imran M, Mushtaq Z, Asghar N (2016) Phytopreventive antihypercholesterolmic and antilipidemic perspectives of zedoary (Curcuma Zedoaria Roscoe.) herbal tea. Lipids Health Dis 15: 1-10.
- Chaturvedi M, Rani R, Sharma D, Yadav JP (2019) Comparison of Curcuma caesia extracts for bioactive metabolite composition, antioxidant and antimicrobial potential. Nat Prod Res 35(18):3131-3135.
- Arya OP, Adhikari P, Pandey A, Bhatt ID, Mohanty K (2022) Health-promoting bioactive phenoli compounds in different solvent extracts of Curcuma caesia Roxb. rhizome from North-East India. Journal of Food Processing and Preservation 46(8): e16805.
- Soto-Vaca A, Gutierrez A, Losso JN, Xu Z, Finley JW (2012) Evolution of phenolic compounds from color and flavor problems to health benefits. J Agric Food Chem 60(27): 6658-6677.
- Bradbury KE, Appleby PN, Key TJ (2014) Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European prospective investigation into cancer and nutrition (EPIC). Am J Clin Nutr 100: 394s-398s.
- Rangel-Huerta OD, Pastor-Villaescusa B, Aguilera CM, Gil A (2015) A systematic review of the efficacy of bioactive compounds in cardiovascular disease: Phenolic compounds. Nutrients 7(7): 5177-5216.
- Vairappan CS, Elias UM, Ramachandram TR, Kamada T (2013) Secondary metabolites from rhizome of Curcuma caesia (Zingiberaceae). Biochem Syst Ecol 48: 107-110.
- Liu W, Zhang Z, Lin G, Luo D, Chen H, et al. (2017) Tetrahydrocurcumin is more effective than curcumin in inducing H22 cell apoptosis via regulation of mitochondria apoptosis pathway in ascetic tumor-bearing mice. Food and Function 8(9): 3120-3129.
- Hanahan D, Weinberg RA (2000) The hallmarks of Cancer. Cell Press 100(1): 57-70.
- Gescher AJ, Sharma RA, Steward WP (2001) Cancer chemoprevention by dietary constituents: A tale of failure and promise. Lancet Oncol 2(6): 371-79.
- Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H (2015) Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci 1348(1): 20-31.
- Araújo JR, Gonçalves P, Martel F (2011) Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res 31(2): 77-87.
- Kang HJ, You, YK, Hong MK, Kim LS (2011) Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. J Korean Med Sci 26(7): 893-899.
- Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30(1): 1-14.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death and Differentiation 25: 486-541.
- Vermeulen K, Bockstaele DRV, Berneman ZN (2003) The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36(3): 131-149.
- Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E (2014) Biology of the cell cycle inhibitor p21(CDKN1A): molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89(2): 155-78.
- Manu KA, Chai TF, The JT, Was LZ, Casey PJ, et al. (2017) Inhibition of isoprenylcysteine carboxylmethyltransferase induces Cell-cycle arrest and apoptosis through p21 and p21-regulated BNIP3 induction in pancreatic cancer. Mol Cancer Ther 16(5): 914-23.
- Ocker M, Schneider-Stock R (2007) Histone deacetylase inhibitors: Signalling towards p21cip1/wafl. The International J Biochemistry and Cell biology 39(7-8): 1367-1374.
- Lincet H, Poulain L, Remy JS, Deslandes E, Duigou F, et al. (2000) The p21 cip1/waf1 cyclin-dependent kinase inhibitor enhances the cytotoxic effect of cisplatin in human ovarian carcinoma cells. Cancer Letters 161(1): 17-26.
- Qin LF, Ng IOL (2001) Exogenous expression of p21WAF1/CIP1 exerts cell growth inhibition and enhances sensitivity to cisplatin in hepatoma cells. Cancer Letters 172(1): 7-15.
- Rottenberg H, Wu S (1998) Quantitaive assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochimica Biophysia Acta 1404(3): 393-404.
- Ashkenazi A (2018) Targeting the extrinsic apoptosis pathway in cancer. Cytokine and Growth Factor Rev 19(3-4): 325-331.
- Shalini S, Dorstyn L, Dawar S, Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ 22(4): 536-530.
- Ma JW, Tsao TCY, His YT, Lin YC, Chen Y, et al. (2016) Essential oil of Curcuma aromatic induces apoptosis in human non-small-cell lung carcinoma cells. Journal of Functional Foods 22: 101-112.
- Franken NAP, Rodermond HM, Stap J, Haveman J, Bree CV (2006) Clonogenic assay of cells in vitro. Nat Protoc 1(5): 2315-9.
- Sokolova V, Fiorino A, Zoni E, Crippa E, Reid JF, et al. (2015) The Effects of miR-20a on p21: Two mechanisms blocking growth arrest in TGF-β-Responsive colon carcinoma. J Cell Physiol 230(12): 3105-3114.
- Bretones G, Delgado MD, León J (2015) Myc and cell cycle control. Biochim Biophys Acta 1849(5): 506-16.
- Chokchaisiri R, Pimkaew P, Piyachaturawat P, Chalermglin R, Suksamrarn A (2014) Cytotoxic sesquiterpenoids and diarylheptanoids from the rhizomes of curcuma elata Rec Nat Prod 8(1):46-50.
- El-Ghorab AH, Nauman M, Anjum FM, Hussain S, Nadeem M (2010) A comparative Study on chemical composition and Antioxidant Activity of Ginger (Zingiber officinale) and Cumin (Cuminum cyminum). J Agric Food Chem 58(14): 8231-8237.
- Hikino H, Konno C, Agatsuma K (1975) Sesquiterpenoids. Part XLVII. Structure, configuration, conformation, and thermal rearrangement of furanodienone, isofuranodienone, curzerenone, epicurzerenone, and pyrocurzerenone, sesquiterpenoids of Curcuma zedoaria. J Chem Soc Perkin Trans 1: 478-484.
- Hikino H, Tori K, Horibe I, Kuriyama K (1971) Sesquiterpenoids. Part XXXVII. Absolute configuration and conformation of zederone, a sesquiterpenoid of Curcuma zedoaria. J Chem Soc, pp. 688-691.
- Jin S, Song C, Jia S, Li S, Chen C, et al. (2017) An integrated strategy for establishment of curcuminoid profile in turmeric using two LC-MS/MS platforms. J Pharmaceut Biomed 132: 93-102.
- KNApSAcK data base.
- Leonel M, Cereda MP (2002) Physicochemical characterization of some starchy tuberoses. Science Tech Food 22: 65-69.
- Li P, Su W, Xie C, Zeng X, Peng W, et al. (2015) Rapid identification and simultaneous quantification of multiple constituents in nao-shuan-tong capsule by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass Spectrometry. J Chromatogr Sci 53(6): 886-897.
- Mahanta BP, Sut D, Kemprai P, Paw M, Lal M, et al. (2019) A1 H‐NMR spectroscopic method for the analysis of thermolabile chemical markers from the essential oil of black turmeric (Curcuma caesia) rhizome: application in post‐harvest analysis. Phytochem Anal 31(1): 28-36.
- Morikawa T (2007) Search for bioactive constituents from several medicinal foods: hepatoprotective, antidiabetic, and antiallergic activities. J Nat Med 61: 112-126.
- Pisarenko O, Shulzhenko V, Studneva I, Pelogeykina Y, Timoshin A, et al. (2015) Structural apelin analogues: mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury. Br J Pharmacol 172(12): 2933-2945.
- Shiobara Y, Asakawa Y, Kodama M, Takemoto T (1986) Zedoarol, 13-hydroxygermacrone and curzeone, theer sesquiterpenoids from Curcuma zedoaria. Phytochemistry 25(6): 1351-1353.
© 2022 Horst MA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






