- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Approaching Obesity and Its Metabolic Changes
Lidiani Figueiredo Santana*
Postdoctoral Student at the Postgraduate Program in Health and Development in the Midwest Region, Brazil
*Corresponding author: Lidiani Figueiredo Santana, Postdoctoral Student at the Postgraduate Program in Health and Development in the Midwest Region, Brazil
Submission: January 07, 2022;Published: February 23, 2022

ISSN:2640-9208Volume6 Issue3
Abstract
For many years, adipose tissue was only considered an energy storage organ, however, it is now known that it plays a key role in the integration of systemic metabolism, and this metabolic function is mediated, in part, by its ability to secrete several proteins, which are called adipokines. Although adipokines are didactically grouped into distinct categories, their actions occur in an integrated manner, causing the participation of TAB in energy homeostasis, immunity, inflammatory response, insulin sensitivity, angiogenesis and blood pressure. When adipose tissue accumulates, the expression of pro-inflammatory cytokines increases, thus causing inflammation and the development of insulin resistance, which are triggering factors for chronic non-communicable diseases.
Mini Review
Adipose cells originate in the embryonic stage from the differentiation of mesenchymal cells, forming adipoblasts and myoblasts that will give rise to pre-adipocytes, then forming adipose tissue, whose main primary functions are the isolation and protection of the organism, storage of Free Fatty Acids (FFA) after food intake and release during the fasting period or high energy requirements to ensure energy supply [1,2]; (Figure 1). Adipose tissue formed from the differentiation of myoblasts, known as brown adipose tissue, is present in large quantities in fetuses and newborns, and in more delimited regions in adults (neck region and around the kidneys). The brown color is due to the large number of mitochondria in this tissue, and favors greater cellular activity regulated by the hormone norepinephrine, being responsible for the regulation of body temperature [3]. White adipose tissue is the best known in the literature, as its main function is to store triglycerides (85-90%), as well as it releases them when in times of high energy demand or prolonged fasting in the form of long-chain fatty acids to be used in energy production, it is found in the subcutaneous (abdominal, gluteal and femoral) and visceral regions, being considered the adipose tissue with the greatest metabolic activity, playing an important role in mechanical protection [4,5].
Figure 1.Adipose tissue formation and pathological complications triggered by the abnormal accumulation of fat in adipocytes.
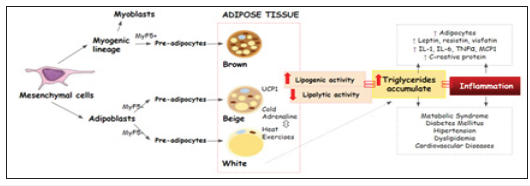
Beige adipose tissue was discovered more recently. Beige cells may not have the same embryonic expression profile in all white fat deposits, and thus may have different origins and, consequently, different characteristics such as number of nerve fibers, vascularization and conditions of environmental exposures [6]. The presence of beige adipose tissue is stimulated by cold and β3-adrenergic receptors and has a key component for energy burning called Uncoupling Protein 1 (UCP1), as well as having the ability to increase its expression, thus favoring increased energy expenditure. There is no specific location, for beige adipose tissue [2]. Mesenchymal cells differentiate into adipoblasts and myoblasts, with the action of MyF5+, transforming them into preadipocytes that later form brown, beige and white adipose tissues. As a result of greater lipogenic activity and less lipolytic activity, triglycerides accumulate in adipocytes, causing hypertrophy and/ or hyperplasia thereof, favoring the elevation of leptin, resistin, vistatin, Interleukin 1 and 6 (IL-1, IL-6), Tumor Necrosis Factor α (TNF-α), monocyte and Macrophage Chemotraction Protein (MCP1) and C-Reactive Protein (CRP), thus intensifying an inflammatory process. Events can trigger factors for the onset of diabetes mellitus, hypertension, dyslipidemia, cardiovascular diseases, among others [7,8].
In this sense, it appears that the adipose tissue has several physiological functions and intense metabolic activity, contributing to energy balance, hormonal regulation (insulin and catecholamines), as well as the nutritional status in conditions of prolonged fasting or increased energy expenditure [9], mediated by the biosynthesis, incorporation and storage of triglycerides from food, as well as by non-lipid substrates, such as carbohydrate, which favors anabolic action, also called lipogenic activity [10]; further the release of stored triglycerides benefit the hydrolysis of TG into long-chain fatty acids or glycerol, to be mobilized into the tissues and promote catabolic action, that is, lipolytic activity [11]. Changes in the dynamics of energy homeostasis, that is, greater lipogenic activity and less lipolytic activity triggers the abnormal accumulation of fat producing hypertrophy and/or hyperplasia of adipocytes, thus naming obesity [5].
Obesity is a chronic health risk and disease, triggered by several etiological factors, including social, behavioral, environmental and genetic factors, defined as an abnormal or excessive accumulation of fat [12]. Over the past three decades, the prevalence of overweight and obesity has increased to 27.5% in adults and 47.1% in children worldwide [13]. The accumulation of adipose tissue is directly related to the expression of several proteins, and when referring to white adipose tissue, it is known that it is capable of producing about 50 cytokines and other molecules that are involved in various physiological or pathological processes acting through autocrine, paracrine and endocrine mechanisms [8,14].
Among the adipokines produced, leptin is a hormone that is directly related to energy metabolism by controlling food intake [7], and obese individuals produce high amounts of this hormone; however, it cannot act on the central nervous system , thus losing its ability to intervene in food consumption, and also has pro-inflammatory activity, that is, its presence causes an increased expression of Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-6 (IL- 6) by monocytes and macrophages [15]. Other cytokines that show increased concentrations in the presence of adipose tissue are Monocyte Chemotactic Protein (MCP-1/ CCL-2), Tumor Necrosis Factor alpha (TNF-α) and Interleukin-6 (IL-6) which are pro-inflammatory, produced by macrophages and lymphocytes, and are directly related to the development of Insulin Resistance (IR) [11,15,16]. Pro-inflammatory cytokines (TNF-α and IL-6) are able to induce the expression of resistin, visfatin and C-reactive protein, which is directly related to IR and is associated with the activation of inflammatory processes through a pathway dependent on the nuclear factor κB (NF-κB) [11,15,17]. Thus, uncontrolled food consumption, the intensification of the inflammatory process caused by increased concentrations of cytokines and insulin resistance, will favor the emergence of comorbidities, which currently represent 7.4% of diabetics and 24.5% of hypertensive individuals who are overweight, all of which increases the risk of morbidity and mortality along with a higher cost to health care [13].
7References
- Kleinert S, Horton R (2019) Obesity needs to be put into a much wider context. Lancet 393(10173): 724-726.
- Kita S, Maeda N, shimomura I (2019) Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. Journal of Clinical Investigation 129(10): 4041-4049.
- Kane H, Lynch L (2019) Innate immune control of adipose tissue homeostasis. Trends Immunol 40(9): 857-872.
- Dominguez MC, Mir JF, Fucho R, Weber M, Serra D, et al. (2016) Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 5(2): 98-118.
- Flaherty SE, Grijalva A, Xu X, Ables E, Nomani A, et al. (2019) A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363(6430): 989-993.
- Lidell M, Betz M, Enerback S (2013) Two types of brown adipose tissue in humans. Adipocyte 3(1): 63-66.
- Zhang F, Hao G, Shao M, Nham K, An Y, et al. (2018) An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metabolism 27(1): 252-262.
- Chung KW, Ha S, Kim SM, Kim DH, An HJ, et al. (2020) PPARα/β activation alleviates age-associated renal fibrosis in sprague dawley rats. The Journals of Gerontology: Series A 75(3): 452-458.
- Noh D, Choi JG, Huh E, Oh MS (2018) Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 10(12): 1998.
- Chin SO, Keum C, Woo J, Park J, Choi HJ, et al. (2016) Successful weight reduction and maintenance by using a smartphone application in those with overweight and obesity. Scientific Reports 6(1): 34563.
- Sarapio E, Souza SK, Model JF, Trapp M, Silva RS (2019) Stanniocalcin-1 and-2 effects on glucose and lipid metabolism in white adipose tissue from fed and fasted rats. Canadian Journal of Physiology and Pharmacology 97(10): 916-923.
- WHO (2020) Obesity and overweight. World Health Organization, pp. 1-2.
- Zobel EH, Hansen TW, Rossing P, Scholten BJV (2016) Global changes in food supply and the obesity epidemic. Current Obesity Reports 5(4): 449-455.
- Petersen C, Nielsen MD, Andersen ES, Basse AL, Isidor MS, (2017) MCT1 and MCT4 expression and lactate flux activity increase during white and brown adipogenesis and impact adipocyte metabolism. Scientific Reports 7(1): 1-13.
- Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nature Reviews Immunology 11(2): 85-97.
- Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, et al. (2018) Brown adipose tissue energy metabolism in humans. Frontiers Endocrinology 9: 447.
- Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, et al. (2016) Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metabolism 23(4): 602-609.
© 2022 Lidiani Figueiredo Santana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






