- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Pears Fortified with Minerals
Patricia Della Rocca*
Center of Chemical Technology, Argentina
*Corresponding author: Patricia Della Rocca, Center of Chemical Technology, Argentina
Submission: October 13, 2021;Published: November 24, 2021

ISSN:2640-9208Volume6 Issue2
Abstract
The main objective was to obtain a ‘snack’ that has a high content of minerals (principally calcium and zinc) through an osmotic dehydration/impregnation process and an extended shelf life that can be achieved by posterior applying a combined drying (convection drying and microwave). The pear slices were immersed in an aqueous solution, which contained lactate calcium 5%m/m, and acetate zinc (0,1%m/m) (49 °Brix). The calcium and zinc concentrations were measured by a flame atomic absorption spectrophotometer and ICP-OES, respectively. The impregnation with minerals was analyzed during time. The samples were osmotic dehydrated/impregnated for 1h. Then, they were dried using a combined method (microwave and hot air convection simultaneous) during another 10-12min to reach 20% of humidity, the value recommended with the Argentinian Food Code. Finally, the osmotic dehydration process was modelled mathematically.
Keywords: Fortified pears; Pears impregnated with Ca and Zn; Osmotic dehydration/impregnation of pears
Introduction
The World Health Organization (WHO) promotes the daily intake of fruits and vegetables
through their “5a day” program in order to create healthy diets. Additionally, the food industry
has shown a growing interest in developing new products to reverse unhealthy eating habits.
In this context, rises the importance of preparing vegetable-based snacks that provide
nutrients, such as dietary fiber, and minerals, like potassium (K), calcium (Ca) and zinc (Zn).
The objective is to add calcium through the consumption of a non-dairy product, that is, a
fortified vegetable. In this way, the ingestion needs of this mineral can be covered by those
who partially or totally restrict the consumption of dairy products due to gastrointestinal
problems. Osmotic dehydration/mineral impregnation improves the characteristics of the
product when it comes to:
a) Nutrition, since metal ions, such as calcium, zinc and iron are incorporated into the
vegetable from the solution.
b) Coloration, because the ascorbic acid and citric acid added by the impregnation solution
act synergistically, preventing enzymatic browning and protecting from subsequent
browning during next drying.
c) Texture, considering that the microstructure of the vegetables is sufficiently preserved
because the incorporated calcium ions react with the pectin’s of the middle lamella, thus
giving rigidity to the cell walls and so reducing the deterioration of the microorganisms.
d) Humidity, since it is decreased but not to the point to be considered a stable shelf product.
e) Consequently, additional posterior drying becomes necessary.
Calcium is not an abundant mineral in most common foods in the Western diet. It is mostly found in dairy products, where calcium is bound to the casein. Human milk has an optimal calcium/phosphorus ratio (2: 1) and factors that enhance absorption (lactose, some peptides and certain characteristics of lipids), therefore the bioavailability of calcium is very high in milk. Some leaf vegetables (like, chard and spinach), fish with bones, some nuts and seeds (almond, sesame, etc.) can be important contributors. However, in the case of vegetables, nuts and seeds, the bioavailability may be low due to the presence of some components, such as oxalates, phytates and fiber that decrease absorption, due to the formation of insoluble complexes. Hence, the necessity to increase the concentration of calcium in vegetables by technology, to maximize then he bio disponible.
The recommended calcium intake per day in children aged 4 to 8 years is 800mg and, in the age range of 9 to 18 years, it is 1100mg for both sexes. In adults 19 to 70 years, it is 800mg in men and 800- 1000mg in women. Zinc is essential for the enzyme activity, which the best known are related to the use of energy, protein synthesis and oxidative protection [1]. Either because it is a constitutive part of the molecule or because it is required as a cofactor. It Zinc plays a fundamental role in stabilizing certain macromolecules (including some receptors) and cell membranes; it also regulates transcription, binding to nuclear proteins. Besides, consumption of zinc is essential in the treatment of maculopathy. The daily needs for an adult between 19 and 70 years of age are 11mg for men and 8mg for women, since its bioavailability is approximately 30% for Western diets. In children between 1 and 3 years, it is 2.5mg and in the age range of 4 to 8 years it is 4mg per day. For both sexes between 9 to 13 years old it is 8mg and between 14 to 18 years in men it is 11mg and in women 9mg daily
Materials and Methods
Material
Peras Packham´s Triumph from the High Valley of Río Negro and Neuquén, which is the main producing and exporting area of pears in Argentina.
Moisture determination
The initial moisture content of the pear was determined according to the standard method [2] by drying in an oven until reaching constant weight.
Determination of soluble solids
The initial soluble solids content of the fresh pear and during the osmotic dehydration/impregnation process were determined with an Abbe refractometer (precision±0.01).
Determination of pulp firmness
Firmness was determined using a Geotech brand manual penetrometer. Mod.:GY-3 (destructive test) consisting of a dynamometer coupled to a stem that is inserted 8mm. in the pulp of the fruit after removing the skin. The cylindrical piercing element used for pears has a diameter of 5/16 inches.
Determination of titratable acidity
The results are expressed in milliliters of 0.1N sodium hydroxide solution per 100g or 100ml of product or in grams of the predominant acid of the product per 100g or 100ml of it. It is considered that 1ml of the 0.1N sodium hydroxide solution is equivalent to: 0.006404g of anhydrous citric acid or 0.006704g of anhydrous malic acid [3].
Final working conditions in the osmotic dehydration/ mineral impregnation experiments
The 5mm thick slices of pears were immersed in an aqueous solution of 40%m/m of sucrose, 5%m/m of calcium lactate, 5%m/m of ascorbic acid, 1%m/m of citric acid and 0.1%m/m of zinc acetate (49 ° Brix) at a temperature of 40 °C. A mass ratio of fortified pear solution/pear mass 4: 1. The container with the pears immersed in the dehydrating solution was placed on an orbital shaker with a thermostat zed chamber at 40 °C with a stirring level of 120rpm. During this process, we worked at atmospheric pressure
Determination of calcium and potassium
The minerals calcium and potassium of the pear samples pretreated by osmotic dehydration/impregnation were analyzed by atomic absorption spectrometry.
Determination of zinc
The zinc determination of the samples was carried out by Inductive Coupling Plasma Atomic Emission Spectroscopy (ICPOES).
Mathematical modelling of osmotic dehydration process
Various literature models were used to adjust the experimental data: [4] modified Page, Henderson [5] and Midilli et al. [6].
Statistical treatment
The adjustment of the kinetic models to the experimental data was carried out by means of non-linear regression, with the Excel 2010 application by the least square’s method and the Solver tool.
Sensory analysis
It was carried out using a consumer test, with an untrained panel, in which 47 people participated, most of whom were nursing and nutrition students from the Universidad del Salvador (Figure 1).
Figure 1.Brix degrees of the pears and the dehydrating solution during the osmotic dehydration/impregnation process.
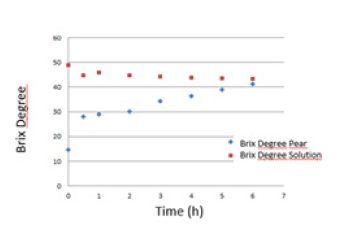
Discussion
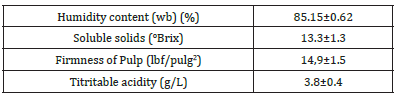
Table 1 shows the results obtained from the characterization of Packham’s Triumph pears in fresh state. The Brix degrees of the pear show a rapid increase after two hours and then a gradual increase until reaching a value similar to that corresponding to the solution at 6 hours after the osmotic dehydration/impregnation process. The calcium and zinc absorption curves during osmotic dehydration/impregnation are presented in Figure 2. In fresh pear, without treatment, the concentration of calcium and zinc is 204mg/kg and 2.1mg/kg, respectively. During the first hour of DO, the absorption of calcium and zinc is 2250mg/kg and 326.44mg/ kg, respectively. As can be seen in this graph, the absorption rate for calcium and zinc is maximum in the first hour of impregnation. After two hours, the increase in absorption of both calcium and zinc continues but occurs very gradually.
Figure 2.Ca and Zn concentration in the pear at different times of osmotic dehydration/impregnation.
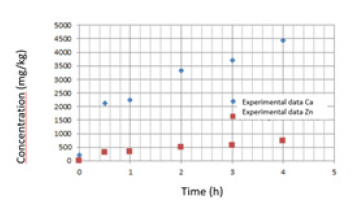
Table 1:Results of the characterization of the pear in fresh state.
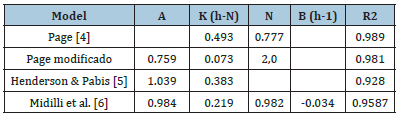
Mathematical modelling Table 2 presents the results obtained by adjusting the mathematical models that describe the experimental data corresponding to the variation in product humidity as a function of time during the osmotic dehydration/ mineral impregnation process. These models are empirical, allowing to obtain the parameters that contribute to the interpretation of the physical process, but their values are valid under similar conditions to those of the experiences. The Page model adjusts the experimental data better. The adjustment can be seen in Figure 3. The Page equation has the advantage of its reduced number of parameters and therefore its mathematical simplicity.
Figure 3.Experimental data of the osmotic dehydration / impregnation process and adjustment of the Page model (H, humidity and subscripts: T: at a certain time, e: in equilibrium, 0: initial.
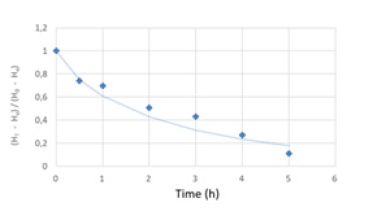
Table 2:Parameter´s values of the models.
Conclusion
Dried and fortified fruits are an alternative to preserve fruit growers’ production. It is estimated that approximately 35-45% of crops are damaged by weather conditions or losses during transport. Generally, damaged fruit is used to make by-products, such as jams. Currently, few companies find in the dehydration of fruits a way to recover part of these losses. In our country, most companies that are dedicated to the dehydration of fruits do so in a small scale. The advantages of the osmotic dehydration/impregnation process were the low energy intake and the possibility to obtain a dehydrated product with an additional mineral value at low cost. The osmotic dehydration/impregnation process improves the nutritional as well as the textural characteristic of the vegetables. The combined subsequent drying is very short in time and allows to reach a stable product considering the possible damage by microorganisms. This treatment also concentrates the potassium that was present in the original pear. The final product has a K content of 193.00mg/100g. It can be considered that the final product is an enriched food, since it reaches between 20-50% of the daily requirements of Ca and Zn per serving, as required by the Argentinian legislation. A dose of product, 25g, covers calcium 22% and zinc 64% of the recommended daily dose.
References
- Cousins RJ (1997) En “Conocimientos actuales sobre nutrición”. In: Ziegler EE & Filer LJ (Ed.), (7th edn), Zinc Capítulo, p. 29.
- Association of Official Analytical Chemists (A.O.A.C) (1996) Official Methods of Analysis (12th edn), AOAC, Washington, USA, pp. 829.
- (1975) AOAC-official methods of analysis of the association of official analytical chemists. (12th edn), Washington DC, USA.
- Page GE (1949) Factors influencing the maximum of air-drying shelled corn in thin layer. Thesis, Purdue University, Indiana, USA.
- Henderson SM, Pabis S (1961) Grain drying theory. En: I Agric Eng Res 6(3): 169-174.
- Midilli A, Kucuk H, Yapar Z (2002) A new model for single layer drying. Drying Technology 20(7): 1503-1513.
© 2021 Patricia Della Rocca. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






