- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Lepidium Sativum Seeds as a Suggested Complex Nutritional Supplement to Treat Biomarkers Related Deficits in Autism
Afaf El-Ansary1,2,3*, Eiman M Ibrahim1 and Ramesa Shafi Bhat4
1 Central Laboratory, Saudi Arabia
2 Autism Research and Treatment Center, Saudi Arabia
3 Therapeutic Chemistry Department, Egypt
4 Biochemistry Department, Saudi Arabia
*Corresponding author:Afaf EA, Central Laboratory, Saudi Arabia
Submission: January 07, 2019;Published: February 07, 2019

ISSN:2640-9208Volume3 Issue3
Abstract
Autism as a neurodevelopmental disorder is characterized by persistent autistic features such as impaired social communication, restricted and repetitive behavior, and intellectual disabilities. As a disorder, autism has several ubiquitous co-morbidities, among which is sleep disorders, epilepsy, attention deficit, and hyperactivity. No effective treatment for the core symptoms of autism is available until now. There is increasing interest in natural products, especially pharmaceutical plants as mono-therapy for the treatment of the core symptoms and co-morbidities of autism. In this review we discuss the safety and effectiveness of Lepidium Sativum seeds as treatment strategy for the early intervention in autism. Up to our understanding of the etiological mechanisms of autism such as oxidative stress, impaired gut microbiota, abnormal lipid metabolism, neuroinflammation, and glutamate excitotoxicity and based on the biomarkers repeatedly recorded to confirm the contribution of these pathways in autism, L. sativum can be suggested as monotherapy. Being an excellent source of non-starch carbohydrates, omega-3 polyunsaturated fatty acids and their precursors, flavonoids, and vitamin E, L. sativum can be suggested as complex supplement to ameliorate the symptoms of autism. Although it is very interesting to find the effectiveness of this plant in treating autism however, convincing pre-clinical data showing efficacy and safety in treating autism is mandatory.
Keywords: Autism; Biomarkers; Lepidium Sativum; Oxidative stress; Gut microbiota; Neuroinflammation; Glutamate excitotoxicity
Introduction
Autism as one of the most common neurodevelopmental disorders is characterized by deficits in social communication, repetitive and restricted behaviors, with multiple sensory abnormalities. Autism is dramatically increasing in prevalence and is now considered an epidemic. There are no objective means to diagnose autism. Diagnosis is made objectively, based on the apparent behavior of the individual [1]. A biomarker is “a distinguishing variable that is accurately measured and assessed as a sign of normal biological manners, pathogenic status, or pharmacological responses to treatment strategy.” They decrease our dependence on patients, caregivers, or clinician scores and are principally important for individuals who are unable to describe their physical or mental problems such as children with autism. Studies of autism biomarkers can enhance our ability to predict abnormal development early in infancy and hence can proceed through early intervention that might be of great help because till now effective medical treatments for the core symptoms of the disorder are still lacking. There is great evidence about the alteration of amino acids, hormones, metabolites and other biomarkers in autistic individuals compared to ageand sex-matched controls. These abnormalities can be observed in the gastrointestinal, immunologic, neurologic, and toxicologi cal systems of the body. In addition, there are unifying etiological mechanisms such as increased susceptibility to oxidative stress, immune dysfunction, glutamatergic dysfunction, and abnormal lipid metabolism. The variances of the biomarkers from the standard present the chance to create a panel of biomarkers that when selectively developed could result in an objective early diagnosis with direct correlation with the severity of autism for everyone [2-8].
Lepidium Sativum
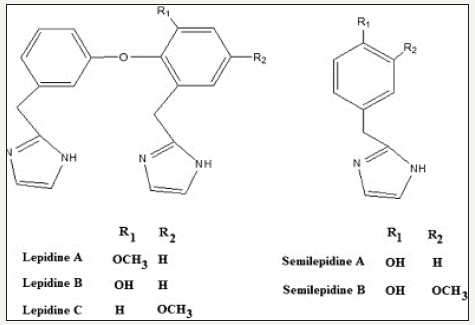
In the search for a natural supplement that can ameliorate most of the abnormalities described above as autistic phenotypes, Lepidium sativum, also known as Garden cress was on the top of the list. L. sativum is an edible plant belonging to the family of mustard. Seeds, leaves and roots of L. sativum are of therapeutic potency; but the plant is mostly cultured for seeds in order to get mucilage for various reasons (Figure 1). In Europe and America, the leaves are used in salad [9]. Seeds have reported the presence of flavonoids, coumarins, sulphur glycosides, triterpenes, sterols and various imidazole alkaloids [10]. The major components of this alkaloid fraction are lepidine and semi-lepidine as shown in Figure 2, a rare group of imidazole alkaloid [9]. The major secondary compounds of this plant are glycosylating [11]. There are red, yellow and black varieties of L. sativum up to their seed color [12]. Additionally, seeds contain 25% of protein, 14-24% of lipids, 33-54% of carbohydrates and 8% of crude fiber [13,14]. The carbohydrates of the L. sativum seeds comprise of 90.0% non-starch polysaccharides and 10% of starch. The seed bran has high dietary fiber content and also it has a high-water holding capacity (74.3%). L. sativum seed contains 20-25% yellowish oil with high percentage of alpha linolenic acid (32-34.0%) [15]. It also has high quantity of polyunsaturated fatty acids (PUFA) (46.8%) and monounsaturated fatty acids (MUFA) (37.6%) and also contains natural antioxidants such as tocopherols and carotenoids which protect the oil from rancidity. Seven imidazole alkaloids in which five lepidine B,C,D,E, and F (dimeric) and two new monomeric alkaloids namely semilepidinoside A and B; and sinapic acid and sinapine were reported in seeds of L. sativum [9,16]. Benzyl isothiocyanate and benzyl cyanide are main volatile constituents of seed. Whereas, β-sitosterol and α-tocopherol are unsaponifiable matter of L. sativum seeds. It also contains mucilages, which upon hydrolysis gives arabinose, galactose, glucose, mannose, xylose and various ironic acids that are the most frequently observed components.
Figure 1:Lepidium Sativum, leaves, flowers, and seeds.

Figure 2:Structures of lepidine and semi lepidine as major components of L. sativum seeds.
Lepidium Sativum as suggested supplement to treat autism
Up to our understanding of the etiological mechanisms of autism, now let us discuss the reasons behind our choice of this plant as recommended supplement to treat this disorder. First, based on the fact that p-cresol levels are significantly higher in blood, urine, and feces of individuals with autism compared to neurotypical children, and it negatively affect the homeostasis of their colonic epithelial cells through the induction of DNA damage [17,18] the 90.0% of non-starch polysaccharides in L. sativum can greatly help in the decrease of p-cresol as deleterious compound. Nutritional intervention studies [19] show that the amount of resistant starch or non-starch carbohydrates in the diet is inversely proportional to the amount of p-cresol in feces and urine. Based on this L. sativum can correct the impaired gut microbiota as confirmed etiological mechanism in autism. It is well documented that while harmful Clostridium species were observed abundant in feces of children with autism, another type of beneficial bacteria, Bifidobacterium, was reduced [20-25]. In the recent work of [26] non-starch carbohydrates (e.g. Fructans, Galactooligosaccharides and Hemicellulose) were suggested as additives to induce the growth of Bifidobacterium in order to mimic breast-fed infant-like microbiota in a formula-fed product. These types of non-starch polysaccharides have been shown to increase the abundance of beneficial bacteria, such as bifidobacteria and lactobacilli, while decreasing counts of potential pathogens, such as Clostridium and Escherichia coli. This can support the use of L. sativum which is rich with non-starch carbohydrates to treat autism.
Second, it is well known that diets rich in ω-6 PUFA are known to have an effect on immune response, as their derivatives such as prostaglandins and leukotrienes are pro-inflammatory. Interestingly, patients with autism have a higher detected ratio of ω-6 PUFA/ ω-3 PUFA compared to healthy control [27]. Immune dysfunction which is often defined in children with autism as well as their family members [28] is frequently related to diets rich in ω-6 PUFA leading to a significantly higher ω-6 PUFA/ ω-3 PUFA as autistic phenotype. [29] reported that individuals with a high ω-6/ ω-3 ratio diet were found to be more vulnerable to the psychological and immunological impact of stress than those with a more balanced ω-6/ω-3 ratio. The ratio of fatty acids namely linoleic acid (LA) and alpha linolenic acid (ALA) as precursors of ω-6 and ω-3 respectively in the diet plays an important role in enrichment of ALA in tissues and further conversion to ω-3 PUFAs (EPA, and DHA). Being one of the richest sources of omega-3 fatty acid and contains 29-34.5% of ALA, L. sativum can be suggested to increase brain EPA and DHA and correct the imbalance of ω-6 PUFA/ ω-3 PUFA ratio repeatedly recorded as marker of autism [30,31].
Flavonoids are polyphenolic compounds that are commonly present in plants and have biological effects on animal cells [32]. Plants containing these bioactive compounds have been used of their favorable effects on human health, decreasing inflammation, stimulating cognition and avoiding cancer [33-36]. Glutamate- mediated excitotoxicity as autistic phenotype is a major factor in neuronal loss. Notably, there is evidence that estrogens are neuroprotective, but their therapeutic use in humans is limited by the increased risk of cancer. Here, we provide evidence that the flavonoid in plants acts as a modulator of estrogen receptors to promote the generation of neurons in vitro and protects against glutamate-mediated neurotoxicity as effectively as the synthetic estrogen estradiol [37]. Moreover, [33-36] highlight the evidence for the potential role of plant flavonoids to inhibit neuroinflammation through an attenuation of microglial activation and associated cytokine release, iNOS expression, nitric oxide production and NADPH oxidase activity. Moreover, they indicate that flavonoid mode of action in the regulation of immune events appear to be mediated by their actions on intracellular signaling pathways, including the nuclear factor-κB (NF-κB) cascade and mitogen-activated protein kinase (MAPK) pathway. As such, flavonoids represent important precursor molecules in the journey to develop of a new generation of drugs capable of lessening neuroinflammation and neurodegenerative disease. Based on our knowledge about the contribution of glutamate excitotoxicity, oxidative stress, NF-κB, and MAPK signaling as etiological mechanisms in autism, and in relation to the flavonoid availability as component of L. sativum, the seed of this plant can be of great help to ameliorate the clinical presentation of all these etiological mechanisms usually appear as social interaction impairment, repetitive behavior, anxiety, epileptic seizures, and hyperactivity.
Children with autism often have diets that are relatively deficient in many nutrients, including vitamin E [38] The use of vitamin E in autism is mostly driven by its antioxidant properties in amending the effects of reactive oxygen species (ROS) or mitochondrial dysfunction as one of the most prevalent metabolic disorders in autism [39,40]. Oxidative damage to proteins has been documented in the brain and other tissues in individuals with autism. Some of the signs that demonstrate the occurrence of abnormal redox metabolism and/or mitochondrial dysfunction in individual with autism are the presence of chronic pain, increased fatigue on the day following infrequent effort, dysautonomia, and severe gastrointestinal disease [41]. Up to this, L. sativum which is rich with vitamin E can be suggested as supplement to ameliorate oxidative stress and mitochondrial dysfunction in individuals with autism.
Conclusion
Up to our knowledge on the pathological mechanisms of autism, our recorded biomarkers presenting the contributed pathways, and our awareness with the medicinal properties and the ingredients of L. sativum, it can be suggested as mono-therapy to ameliorate the core symptoms of autism and the concomitant co-morbidities. Of course, pre-clinical data is highly recommended.
References
- Ratajczak HV (2011) Theoretical aspects of autism: biomarkers-a review. J Immunotoxicol 8(1): 80-94.
- Ford TC, Akel AA, Crewther DP (2019) The association of excitation and inhibition signaling with the relative symptom expression of autism and psychosis-proneness: Implications for psychopharmacology. Prog Neuropsychopharmacol Biol Psychiatry 88: 235-242.
- Montgomery AK, Shuffrey LC, Guter SJ, Anderson GM, Jacob S, et al. (2018) Maternal serotonin levels are associated with cognitive ability and core symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 57(11): 867-875.
- Yang XL, Liang S, Zou MY, Sun CH, Han PP, et al. (2018) Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Res 259: 229-235.
- Rose DR, Yang H, Serena G, Sturgeon C, Ma B, et al. (2018) Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun 70: 354-368.
- Zhou H, Zhao W, Ye L, Chen Z, Cui Y (2018) Postnatal low-concentration arsenic exposure induces autism-like behavior and affects frontal cortex neurogenesis in rats. Environ Toxicol Pharmacol 62: 188-198.
- Jafari T, Rostampour N, Fallah AA, Hesami A (2017) The association between mercury levels and autism spectrum disorders: A systematic review and meta-analysis. J Trace Elem Med Biol 44: 289-297.
- Grayaa S, Zerbinati C, Messedi M, HadjKacem I, Chtourou M, et al. (2018) Plasma oxysterol profiling in children reveals 24 hydroxycholesterols as a potential marker for Autism Spectrum Disorders. Biochimie 153: 80- 85.
- Maier UH, Gundlach H, Zenk MH (1998) Seven imidazole alkaloids from Lepidium Sativum. Phytochemistry 49(6): 1791-1795.
- Radwan HM, Missiry MME, Said WMA, Ismail AS, Shafeek KAA, et al. (2007) Investigation of the Glucosinolates of Lepidium Sativum Growing in Egypt and Their Biological Activity. Res J Medicine & Med Sci 2(2): 127-132.
- Gill V, Macleod AJ (1980) Studies on glucosinolate degradation in Lepidium Sativum seed extracts. Phytochemistry 19(7): 1369-1374.
- Nuez F, Hernandez-Bermejo JE (1994) In: Bremejo JEH, Leon J (Eds.), Neglected Crops: 1492 From a Different Perspective, Plant Production and Protection Series No. 26, FAO, Rome, Italy, pp. 303-332.
- Arkroyd WR, Gopalan C, Balasubramanian S (1963) The nutritive value of Indian foods and the planning of satisfactory diets. Spec Rep Ser Indian Counc Med Res 42: 1-255.
- Mathews S, Singhal RS, Kulkarni PR, Die Nahrung (1993) Some physicochemical characteristics of Lepidium Sativum (haliv) seeds. Molecular Nutrition Food Research 37(1): 69-71.
- Diwakar BT, Dutta PK, Lokesh BR, Naidu KA (2010) Physicochemical Properties of Garden Cress (Lepidium Sativum L.) Seed Oil. J Am Oil Chem Soc 87(5): 539-548.
- Nayak PS, Upadhyay A, Dwivedi SK, Rao S (1952) Electron J Environ Agric Food Chem 11(2012): 156-162.
- Selmer T, Andrei PI (2001) P-hydroxyphenylacetate decarboxylase from clostridium difficile. A novel glycyl radical enzyme catalysing the formation of pcresol. Eur J Biochem 268(5):1363-1372.
- Andriamihaja M, Lan A, Beaumont M, Audebert M, Wong X, et al. (2015) The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med 85: 219-227.
- Chen BX, Morioka S, Nakagawa T, Hayakawa T (2016) Resistant starch reduces colonic and urinary p-cresol in rats fed a tyrosine-supplemented diet, whereas konjac mannan does not. Biosci Biotechnol Biochem 80(10): 1995-2000.
- Song YL, Liu CX, Finegold SA (2004) Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 70(11): 6459-6465.
- Finegold SM, Molitoris D, Song YL, Liu CX, Vaisanen ML, et al. (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35(suppl 1): S6-S16.
- Parracho H, Bingham MO, Gibson GR, McCartney AL (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54(Pt 10): 987-991.
- Angelis MD, Piccolo M, Vannini L, Siragusa S, Giacomo AD, et al. (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLos One 8(10): e76993.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT , et al. (2011) Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 77(18): 6718-6721.
- Adams JB, Johansen LJ, Powell LD , Quig D, Rubin RA (2011) Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11: 22.
- Ackerman DL, Craft KM, Townsend SD (2017) Infant food applications of complex carbohydrates: Structure, synthesis and function. Carbohydr Res 437: 16-27.
- Vancassel S, Durand G, Barthélémy C, Lejeune B, Martineau J, et al. (2001) Plasma fatty acid levels in autistic children. Prostaglandins Leukotrienes Essential Fatty Acids 65(1):1-7.
- Goines P, Water JV (2010) The immune system’s role in the biology of autism. Current Opinion Neurology 23(2): 111-117.
- Glaser JKK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S (2007) Depressive symptoms, omega-6: omega-3 fatty acids, and inflammation in older adults. Psychosomatic Medicine 69(3): 217-224.
- Diwakar BT, Dutta PK, Lokesh BR, Naidu KA (2008) Bio-availability and metabolism of n-3 fatty acid rich garden cress (Lepidium Sativum) seed oil in albino rats. Prostaglandins Leukot Essent Fatty Acids 78(2): 123- 130.
- Ramprasad TR, Baskaran V, Sambaiah K, Lokesh BR (2010) Lower efficacy in the utilization of dietary ALA as compared to performed EPA and DHA on long chain n-3 PUFA levels in rats. Lipids 45(9): 799-808.
- Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5): 270-278.
- Spencer JP, Vafeiadou K, Williams RJ, Vauzour D (2012) Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med 33(1): 83-97.
- Williams RJ, Spencer JP (2012) Flavonoids, cognition and dementia: actions, mechanisms and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 52(1): 35-45.
- Sokolov AN, Pavlova MA, Klosterhalfen S, Enck P (2013) Chocolate and the brain: neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci Biobehav Rev 37(10): 2445-2453.
- Spencer JP, Mohsen MMAE, Minihane AM, Mathers JC (2008) Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 99(1): 12-22.
- Souza CDS, Grangeiro MS, Pereira EPL, Santos CCD, Silva ABD, et al. (2018) Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. Neurotoxicology 65: 85-97.
- Bauset SMJ, González AL, Zazpe I, Sanchis AM, Varela MMS (2017) Comparison of nutritional status between children with autism spectrum disorder and typically developing children in the Mediterranean Region (Valencia, Spain). Autism 21(3): 310-322.
- Giulivi C, Zhang YF, Klusek AO, Inta CR, Wong S, et al. (2010) Mitochondrial dysfunction in autism. JAMA 304(21): 2389-2396.
- Frye RE, Rossignol DA (2016) Identification and treatment of pathophysiological comorbidities of autism spectrum disorder to achieve optimal outcomes. Clin Med Insights Pediatr 10: 43-56.
- Napoli E, Wong S, Picciotto IH, Giulivi C (2014) Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 133(5): 1405-1410.
© 2018 Afaf EA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






