- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Phytochemical Composition and Nutritional Properties of Non-Diary Probiotic Beverages
Isitua CC*, Olagbemide PT and Onwuegbunam OV
Department of Biological Sciences, Afe Babalola University, Nigeria
*Corresponding author:Isitua CC, Department of Biological Sciences, Afe Babalola University, Ado-Ekiti, Nigeria
Submission: September 18, 2018;Published: October 11, 2018

ISSN:2640-9208Volume3 Issue1
Abstract
Functional foods serve nutrients and physiologically active components for a healthy living. Though dairy is the ideal food matrix for probiotics but because it might cause allergy in certain segments of the population, the trend of non-dairy probiotics is growing among consumers. The aim of this research was to provide a non-dairy probiotic drink to attend people that cannot eat dairy products due to lactose intolerance and determine its phytochemical and proximate composition. Proximate analysis (moisture, crude fiber, crude ash, protein, carbohydrate and fat and oil) and Photochemical analyses on Steroids, Cardiac glycosides, phenols, tannins, terpenoids, alkaloids, flavonoids, Saponins and reducing sugars were determined in triplicates according to standard methods. The statistical analysis was conducted using the SPSS software version 18.0 (Chicago, IL, USA). Proximate composition of probiotic juice showed increase in level of moisture content, reduction in level of fat and absence of crude fiber. The probiotic beverages showed positive for cardiac glycosides, Alkaloids, Flavonoids and reducing sugars but showed negative for steroids, phenol, tannins, terpenoids, glycosides and saponins. These juice samples were found to be nutritionally and phytochemically rich.
Introduction
Foods have many aims of satisfying hunger and providing the necessary nutrients for humans, promoting a state of physical and mental well-being, improving health, preventing and/or reducing nutrition-related diseases. Nowadays, food is no longer considered by consumers only in terms of organoleptic properties and immediate nutritional needs, but also in terms of their ability to provide specific physiological benefits in management and prevention of diseases beyond their basic nutritional value. Foods that provide a health benefit beyond basic nutrition are known as functional foods [1] and is estimated that a 20% reduction in healthcare expenditure could be achieved through their widespread consumption. Pro-biotification of foods is one of the methods used to produce such new functional foods. Currently, the largest segment of the functional food market is dominated by healthy food products targeted towards improving the balance and activity of the intestinal microflora [2]. Probiotic products are known to have medical uses in particular [3] and for a variety of gastrointestinal conditions and in preventing antibiotic-associated diarrhea. Apart from people consuming probiotics as fermented food products, now probiotics are added as supplements [4]. The mechanism of action of probiotics with anti-microbial properties maybe due to the production of bacteriocins such as niacin [5] or lowering the pH by producing acidic compounds like lactic acid [6]. Probiotic strains compete with other infectious bacteria for nutrients and cell-surface and help toward them off by inhibiting their colonization [7]. A few strains are also known to produce active enzymes which inhibit other pathogenic bacteria [8]. In addition, reduction of serum cholesterol, alleviation of lactose intolerance, reduction of cancer risk, resistance to enteric pathogens, etc. are some of the documented benefits of ingestion of probiotics [9].
Dairy fermented products have been traditionally considered as the best carriers for probiotics; but, nowadays, up to 70% of the world population is affected by lactose-intolerance and also the use of milk-based products may be limited by allergies, cholesterol diseases, dyslipidemia, and vegetarianism. Furthermore, dairy products especially to yoghurt, can only be afforded by the privileged few in the developing countries. Thus, to satisfy the food needs of all groups of people, non-dairy probiotic product becomes an obvious choice [10]. Therefore, several raw materials have been extensively investigated to determine if they are suitable substrates to produce novel non-dairy functional foods [11]. Fruit juices including mango, rosehips and strawberry are being employed as carriers of probiotic strains [12]. The market available non-dairy probiotic products are juice, fruits and vegetables, cereal based products, chocolate-based products, meat-based products and many others [13-15]. Fermented fruits and vegetables can be used as a potential source of probiotics as they harbor several lactic acid bacteria such as Lactobacillus plantarum, L. pentosus, L. brevis, L. acidophilus, L. fermentum, Leuconostoc fallax, and L. mesenteroides. Juices combine nutritional effects with the added value of a healthy benefit from a probiotic. Many authors reported on the effects of juices on health; for example, Sutton et al. [16] demonstrated that aqueous extracts of kiwifruit and avocado had very low cytotoxicity and high anti-inflammatory activity in a Crohn’s gene-specific assay. Non-aqueous extracts of kiwifruit, blueberry and avocado had similarly high anti-inflammatory activity, with slightly higher cytotoxicity than the aqueous extracts. Fruits and vegetables have been suggested as suitable media for cultivation of probiotics because they inherently contain essential nutrients; high amount of vitamins, mineral and polyphenolic compounds, free from allergens and easily available with attractive appearance and taste [17,18]. The aim of this research was to provide a non-dairy probiotic drink to attend people that cannot eat dairy products due to lactose intolerance and determine its phytochemical and proximate composition.
Materials and Methods
The materials applied in probiotic beverage production process were crude extracts of oranges, banana, apple, carrot, watermelon, coconut water, pineapple, ginger, Moringa leaf powder and Roselle flowers. The mixture of the fruits above were provided as ratios 20%, 30% and 40% and reached volume 1000ml by distilled water and the specimen was kept in the water bath for pasteurization at 80 °C for 5minutes [19]. Proximate analysis (moisture, crude fiber, crude ash, protein, carbohydrate and fat and oil) were determined using standard method [20]. Photo chemical analyses were carried out on Steroids, Cardiac glycosides, phenols, tannins, terpenoids, alkaloids, flavonoids, Saponins and reducing sugars according to standard methods [21,22].
Statistical analysis
All experimental measurements were carried out in triplicate. The statistical analysis was conducted using the SPSS software version 18.0 (Chicago, IL, USA). Analysis of variance (ANOVA) was performed using the ANOVA procedure. Significant differences (P< 0.05) between means were determined by Duncan`s multiple range test.
Result
Phytochemical Constituents of the Probiotic Beverage
The phytochemical constituents of the probiotic beverage before and after fermentation was shown in Table 1 & 2. The probiotic beverages showed positive for cardiac glycosides, Alkaloids, Flavonoids and reducing sugars but showed negative for steroids, phenol, tannins, terpenoids, glycosides and saponins. The proximate composition of the probiotic beverages after fermentation was shown in Table 1. The highest moisture content level was 96.34% in 30% (con) and the lowest was 96.30% in 40% (106). The carbohydrate content was relatively low. The highest was 2.39% in 40% (106) and the lowest was 20% (107). There was no crude fiber recorded as fruits do not contain crude fiber. The crude fat was relatively low. The highest crude fat was 0.101% in 40% (106) and the lowest was 0.062% in 30% (106). The highest crude ash was 0.90% 40% (107) and the lowest was 0.82% in 30%(con) and the highest protein was 0.333% in 40% (107) and the lowest protein was 0.300% in 40% (con). The highest energy value was 11.7Kcal/100g and the lowest was 11.3Kcal/100g.
Table 1:Phytochemical constituents of the Probiotic beverages before fermentation.
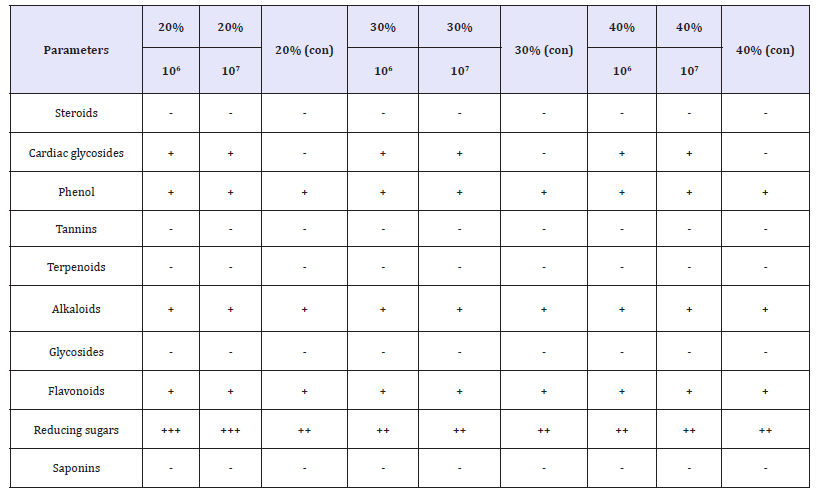
Table 2:Phytochemical constituents of the Probiotic beverages after fermentation.
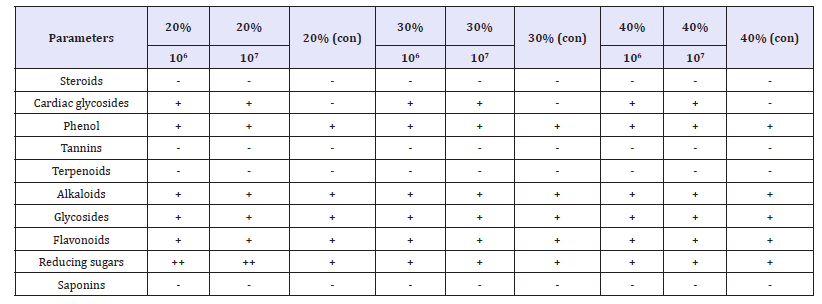
Keys: con= control, - = not detectable, + = Low concentration, ++ = Medium concentration, +++ = High concentration
Discussion
Probiotics are live microorganisms that contribute a beneficial effect on the host when administered in proper amounts. Multiple reports have described their health benefits on gastrointestinal infections, antimicrobial activity, reduction in serum cholesterol, immune system stimulation, improvement in lactose metabolism, anti-mutagenic properties, anti-carcinogenic properties, anti-diarrheal properties [23-25].
Phytochemicals are non-nutritive plant chemicals possessing varying degrees of disease-preventive properties. They are invaluable sources of raw materials for both traditional and orthodox medicine. The presences of cardiac glycosides are known to play a major role in heart muscles by inhibiting Na+ and K+ pump that increase the availability of sodium ions and calcium ions to heart muscles which improves cardiac output and reduce heart distension. Thus, they are used in the treatment of congestive heart failure and cardiac arrhythmia. Flavonoids are the group of phenolic compounds that act as primary antioxidants and posse’s antimicrobial, anti-inflammatory, anti-allergic, anticancer, antineoplastic activity, and for the treatment of intestinal disorders. Alkaloids are one of the largest groups of phytochemicals that have led to the invention of powerful pain killer medications. Results of the phytochemical constituents in the sample materials as shown in Table 1 & 2 implied that the phytochemical analysis of the beverages was done before and after probiotication. The samples had high concentration of reducing sugars. Flavonoids, alkaloids, phenols and cardiac glycosides were also present. These results agree in part with the findings of who reported the presence of reducing sugars, phenols and flavonoids in citrus juice. These differences may be due to differences in species and geographical location.
Table 3:Proximate composition of the beverages.
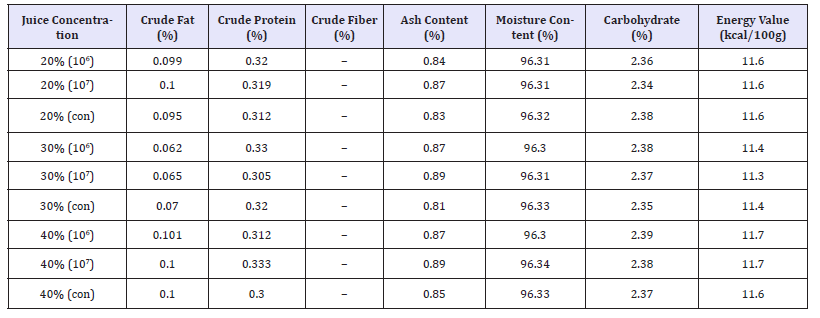
Keys: con= control, - = not detectable, + = Low concentration, ++ = Medium concentration, +++ = High concentration
Energy value of a food measures its value to the body as a fuel and it measures the inherent chemical energy inherent in the bonds of the organic compounds of foods such as their protein, carbohydrate and fat constituents as well as minor constituents such as organic acids. The study of ash content is very important to the extent that it provides an insight into the nutritionally important inorganic mineral elements as shown in Table 3 reported that the ash content of a food sample gives an idea of the mineral elements present in the food sample [26]. It has been reported by Salvi et al. [27] that proximate compositions of fruits vary with location where the fruits are grown. The moisture content of the probiotificated juice was relatively high because of the state of the sample which is in liquid form. There was absence of crude fiber and very little fat in the sample. 40% (107) had the highest moisture content of 96.34% and 30% (106) has the lowest moisture content of 96.30%.
According to David Asante [28], the highest moisture content was 93.49% and the lowest was 92.58% which agrees to my result. There was no significant difference (P< 0.05) between the percentage moisture content of the nine juice samples. This result shows that statistically the percentage moisture content of the nine probiotic beverages drinks were the same at 95 % confidence level. The high-water content helps the body as the body does not need to use some of its own water to digest them. This means that the body uses less energy and resources to digest and can then assimilate all the nutrients much faster. Less pressure is therefore put on the digestive system [28]. The 40% (107) sample had the highest crude protein of 0.333% and 40% (con) had the lowest crude protein of 0.300%. This result disagrees with the findings of [29].
There was no significant difference in the percentage crude protein of the nine beverage samples. This result shows that statistically the percentage crude protein of the nine probiotic beverages drinks were the same at 95% confidence level. The highest carbohydrate and crude fat were 2.38% and 0.101% respectively; the lowest was 2.35% and 0.065% respectively. These findings are not in agreement with that of [30]. This could be since it was only one kind of fruit and one type of probiotic organism that was used in that study compared to the ten different fruits and vegetables with ten different organisms used in this study. The energy value of the nine juice concentrations are not significantly different from each other (P>0.05). The beverages had very low calories ranging from 11.7 to 11.3Kcal/100g. This could be since the fruits and vegetables used for this research had low calories therefore manifesting in the mixture. There was no significant difference in the energy content of the nine beverages. This result shows that statistically the energy content of the nine probiotic beverages drinks were the same at 95% confidence level. Thus, fruit juices represent a promising carrier for probiotic bacteria.
References
- Serafini M, Stanzione AS, Foddai S (2012) Functional foods: traditional use and European legislation. Int J Food Sci Nutr 63 (suppl 1): 7-9.
- Saarela M, Lähteenmäki L, Crittenden R, Salminen S, Mattila ST (2002) Gut bacteria and health foods the European perspective. Int J Food Microbiol 78(1-2): 99-117.
- Ringdahl EN (2000) Treatment of recurrent vulvovaginal candidiasis. Am Fam Physician 61(11): 3306-3317.
- Ranadheera R, Baines S, Adams M (2010) Importance of food in probiotic efficacy. Food research international 43(1): 1-7.
- Yateem A, Balba MT, Al Surrayai T, Al Mutairi B, Al Daher R (2008) Isolation of lactic acid bacteria with probiotic potential from camel milk. Int J Dairy Sci 3: 194-199.
- Psomas E, Andrighetto C, Litopoulou TE, Lombardi A, Tzanetakis N (2001) Some probiotic properties of yeast isolates from infant feces and feta cheese. Int J Food Microbiol 69(1-2): 125-133.
- Piard JC, Desmazeaud M (1991) Inhibiting factors produced by lactic acid bacteria: Oxygen metabolites and catabolism end products Bacteriocins and other antibacterial substances. Le Lait INRA Editions 71: 525-541.
- Gotcheva V, Hristozova E, Hristozova T, Guo M, Roshhoya Z et al. (2002) Assessment of potential probiotic properties of lactic acid bacteria and yeast strains. J Food Biotechnology 16(3): 211-225.
- Gilliland SE (1990) Health and nutritional benefits from lactic acid bacteria. FEMS Microbiology Rev 87(1): 175-188.
- Sethi S, Tyagi S, Anurag RK (2016) Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol 53(9): 3408-3423.
- Perricone M, Bevilacqua A, Corbo MR, Sinigaglia M (2014) Technological characterization and probiotic traits of yeasts isolated from Altamura sourdough to select promising microorganisms as functional starter cultures for cereal-based products. Food Microbiol 38: 26-35.
- Beverage World (2009) A cultured trend.
- Blandino A, Al Aseeri ME, Pandiella SS, Cantero D, Webb C (2003) Cereal based fermented foods and beverages: Review. Food Research International 36: 527-543.
- Farnworth ER, Mainville I, Desjardins MP, Gardner N, Fliss I, et al. (2007) Growth of probiotic bacteria and bifido bacteria in a soy yogurt formulation. Int J Food Microbiol 116(1): 174-181.
- De Bellis P, Valerio F, Sisto A, Lonigro SL, Lavermico P (2010) Probiotic table olives: microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus Paracasei IMPC2 in an industrial plant. Int J Food Microbiol 140(1): 6-13.
- Sutton KH (2007) Considerations for the successful development and launch of personalized nutrigenomic foods. Mutation Research 622(1- 2): 117-121.
- Pereira DIA, Gibson GR (2002) Effects of consumption of probiotics and prebiotic on serum lipid levels in humans. Crit Rev Biochem Mol Biol 37(4): 259-281.
- Luckow T, Sheehan V, Fitzgerald G, Delahunty C (2006) Exposure health information and flavored masking strategies for improving the sensory quality of probiotic juice. Apetite 47(3): 315-325.
- Mousavi Z, Mousavi S, Razavi S, Emam DZ, Kiani H (2011) Fermentation of pomegranate juice by probiotic lactic acid bacteria. World Journal of Microbiology and Biotechnology 27(1): 123-128.
- AOAC (2012) Official method of analysis: Association of Official Analytical Chemists. Washington DC, USA.
- Evans WC (2002) Trease and Evan’s pharmacognosy. (5th edn), Harcourt Brace and Company, p. 36.
- Trease GE, Evans WC (1989) A Text Book of Pharmacognosy. Academic Press. Bacilluere Tinal Limited London. (13th edn), pp. 22 -40.
- Kechagia M, Basoulis D, Konstan TS, Dimitriadi D, Gyftopoulou K, et al. (2013) Health benefits of probiotics. A Review. Hindawi Publishing Corporation. ISRN Nutr 2013: 481651.
- Boon YW, Suyang RA (2014) The health benefits of probiotics. Journal Sains Kesihatan Malaysia 12 (2): 41-44.
- Kaur A, Arora M, Pandove G (2014) Probiotics and its health benefits. Journal of Global Bio sciences 3(3): 686-693.
- Bhattacharjee S, Sultana A, Sazzard M, Islam M, Ahtashom M, et al. (2013) Analysis of the proximate composition and energy values of two varieties of onion (Allium cepa L.) bulbs of different origin: A comparative study. International Journal of Nutrition and Food Science 2(5): 246-253.
- Salvi MJ, Rajput JC (1995) Handbook of science and technology: production composition storage and processing. Marcel Dekker Inc. 207 madison Avenue, New York, 10016, USA, pp. 171-181.
- David Asante D (2010) Product development of sports drink using coconut water and pineapple juice. Thesis. Kwame Nkrumah University of Science and Technology, Kumasi, West Africa.
- Kwenin WK, Wolli M, Dzomeku DM (2011) Assessing the nutritional value of some African indigenous green leafy vegetables in Ghana. Journal of Animal & Plant Sciences 2(2): 16-23.
- Rafiq S, Sharma V, Nazir A, Rashid R, Sofi SA, et al. (2016) Development of probiotic carrot juice. Journal of Nutrition and Food Sciences 6: 41-45.
© 2018 Isitua CC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






