- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Effect of Storage on Protein Composition of Fermented Soybean (Glycine Max) Seed by Bacillus Subtillis
Modupe Elizabeth Ojewumi*, Adebola Oyinade Odubiyi and James Abiodun Omoleye
Department of Chemical Engineering, Covenant University, Nigeria
*Corresponding author:Modupe Elizabeth Ojewumi, Department of Chemical Engineering, Covenant University, Canaan land, Ogun State, Nigeria
Submission: May 24, 2018;Published: August 20, 2018

ISSN:2640-9208Volume2 Issue4
Abstract
The effect of storage (shelf life) on percentage protein composition of stored fermented soybean seed was studied. Samples were fermented with Bacillus substilis (as a starter culture) and without (natural fermentation) for 3 days. Two methods of preservation were used, namely; oven drying and salting. Each sample was oven dried within 4-24hours and the percentage moisture content removed was calculated. 1-3% salt was also added to the fermented samples to monitor the effect of salting on the shelf life. Constant weight was obtained from 12hours of drying and about 0% moisture content which is very close to total dryness was obtained in both fermented soybean samples. Samples were monitored for two weeks. The protein content reduced with storage duration while pH moved towards acidic medium.
Keywords: Fermentation; Glycine max; Shelf life; Physiology; Bacillus subtillis
Introduction
Soyabean (Glycine max) or soybean is a species of legume, a native to East Asia and is widely grown for its edible bean which has innumerable uses [1]. Like most plants, soybeans grow in distinct morphological stages as they develop from seeds into fully mature plants. The first stage of growth is germination, a method which first becomes apparent as a seed’s radical emerges [2]. Like many legumes, soybeans can fix atmospheric nitrogen [3]. Soybean seeds are extremely high in protein content. On average, dry soybean contains roughly 40% protein, 35% carbohydrate, 20% soybean oil, and 5% ash (non-aqueous, metal oxides). Therefore, soybean has the highest protein content among legume species. Soy protein is a heat-stable protein, thus allowing soy seeds to undergo high temperature cooking and fermentation, without destroying the entire chemical composition of the soybean.
The beans contain significant amounts of phytic acid, dietary minerals and B vitamins [4]. Soy vegetable oil, used in food and industrial applications, is another product of processing the soybean crop. Traditional non-fermented food uses of soybeans include soy milk from which tofu and tofu skin are made. Fermented soy foods include soy sauce, fermented bean paste, natto and tempeh [5,6].
Fermentation is the chemical breakdown of substance by bacteria, yeast or other microorganism into alcohol, carbon dioxide or organic acids [7]. Fermentation may also decrease or eliminate anti-nutritional constituents [8-11]. Overall, the nutritional quality of the fermented product is improved. Fermented Soya has been shown to inhibit E coli infection [12], minimize fluid losses in ETEC-infected small intestinal segments of piglets [12-16], and shorten the duration of diarrhoea when supplemented to the diet of malnourished children [17,18].
There seems to be a general agreement on the spore-forming Bacillus species as the main fermentation organisms [7,19-25]. During the fermentation of soya bean with systematic investigation showed that Bacillus subtillis is the most dominant bacterium responsible for the fermentation [5,26]. Literature revealed that some species of bacillus such as Bacillus lichenioformis, Bacillus megaterium, L. mesenteriodes and Staphylococcus are also found in the fermented condiment (Iru). In a study conducted on the fermentation of Iru, it was found that Gmelinaarborea as well as banana leaves accelerated fermentation of seeds, while also bringing an increase in protein and crude fat contents with corresponding decrease in carbohydrate [27].
Materials and Method
Source of raw materials
Soya bean seeds were bought from open market. The starter cultures used were freshly prepared in the Microbiology laboratory, Covenant University, Ota, Nigeria.
Preparation of seed for fermentation process
The seeds were processed according to method [28,29]{Ojewumi, 2017 #26;Ojewumi, 2018 #181}.
Preparation of starter culture
Previously isolated Bacillus subtilis was used in solid inactive form. This was activated by taking some of the bacteria with a loop and mixing it with a freshly prepared nutrient broth and incubated for 24hours.
pH determination
Method [30,31] was used for the determination of pH. Moisture content determination Method [6] was used. Sample was oven dried after fermenting for 3 days and dried between 4 to 24hrs with four hours interval. The percentage moisture content was calculated until constant weight was obtained from 12-24hrs with 0% moisture content. Samples were cooled in a desiccator and the final weights were recorded and stored in an air tight container.
Proximate analysis
Determination of % Protein composition using method [5] and moisture content was carried out in this research. The analysis was done weekly for 2weeks (Figure 1).
Result
Figure 1:The effect of storage duration on pH of oven dried naturally fermented Soybean
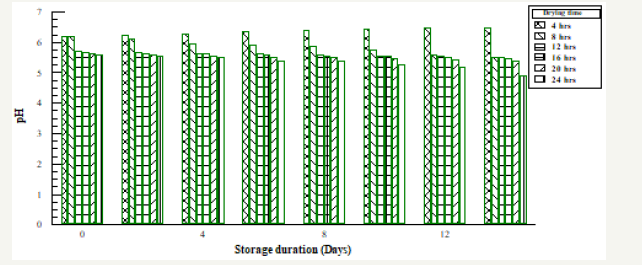
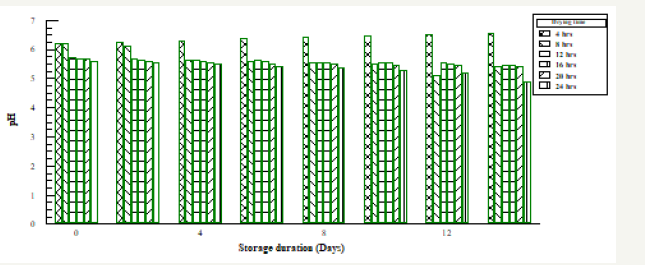
Figure 2:The effect of sorage duration on pH of Bacillus subtilis fermented Soybean.
Discussion
Percentage moisture content
The result of this research work show that oven drying improves the shelf life of soybean. Effect of moisture content on its deterioration was carried out and the bacteria responsible for deterioration need moisture for their metabolism. The samples dried for up to 12-16 hours showed to have better preservation of the functional properties. The ones dried for 4- 8 hours still had moisture in them, which increased its deterioration while the ones dried for longer than 16 hours had no moisture in them anymore but protein concentration were found to be a little low, which could indicate that due to high temperature some of this properties of the seeds have been lost (Figure 2).
Figure 3:Protein analysis for Bacillus subtilis fermented oven dried samples.
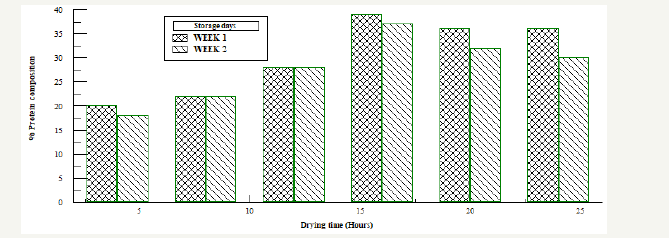
Figure 4:Protein analysis for naturally fermented oven dried samples.
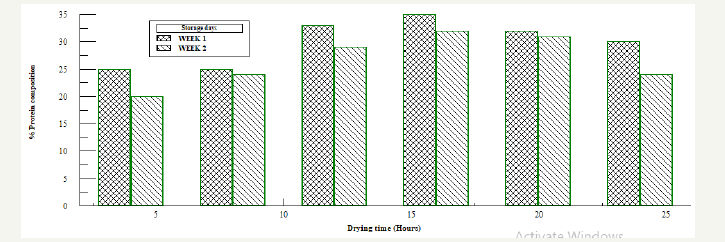
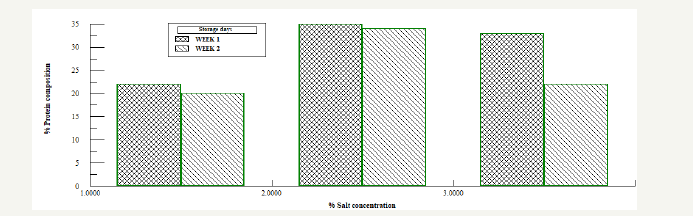
Figure 5:Protein analysis for Bacillus subtilis fermented and salted samples.
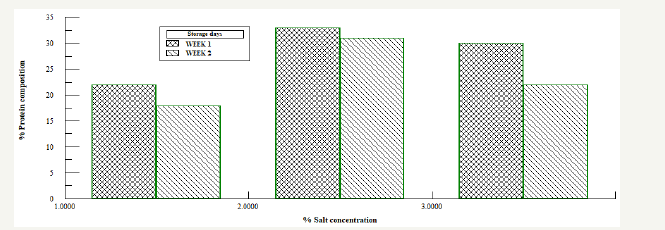
Figure 6:Protein analysis for Bacillus subtilis fermented and salted samples.
pH analysis
Table 1: Percentage moisture content removed for naturally fermented oven dried samples.
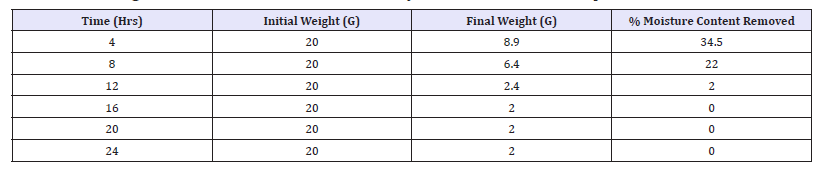
Table 2: Percentage moisture content removed for Bacillus Subtilis fermented oven dried samples.
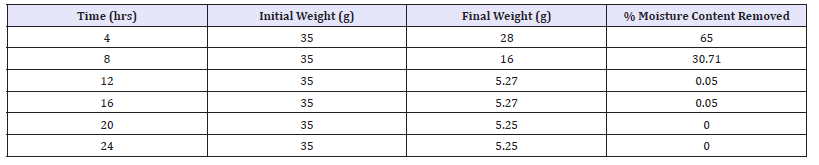
Table 1 & 2 above shows the effect of storage duration on the pH of both fermented samples. The higher the pH the lower the risk and rate of spoilage, since microorganisms finds it difficult to strive in an acidic medium. Increase in pH towards the acidic range was recorded for drying durations 8-24 hours while increase in pH towards alkaline medium was noticed at 4 hour. The increase in pH observed in this study is an indication that fermentation still continued after the processing period of soybean to a protein condiment (post fermentation operation). This confirmed that the organisms responsible for the fermentation are still present at consumption [6].
Protein Analysis
Oven dried samples fermented using Bacillus subtillis was monitored for two weeks and protein analysis were carried out every week on each sample. The protein concentration was determined by tracing the absorbance on the standard absorbance-concentration protein graph and the values were multiplied by 10 using the conversion formula, Figure 3. From week 1 sample dried for four hours showed a higher protein concentration but as storage time increased the concentration of the protein began to vary, it increased and then decreased for each sample all through the storage period. Sample dried for 16 hours had a more stable protein concentration with storage although the concentration varied too with storage time compared to the samples dried at 4,8 12,20 and 24 hours.
Also on naturally fermented oven dried samples Figure 4, the results shows that protein concentration for each of the dried samples were higher compared to the bacillus fermented oven dried samples. From the graph the protein concentration varied with storage time. Samples dried for 16 hours had the higher protein concentration compared to the samples dried for 4, 8, 12, 20 and 24 hours. Samples with and without starter culture was divided into three, and salt was added as a preservative to each of the sample in the ratio 1%: 2%: 3%, Figure 5 & 6. It was observed that after the first week of storage, sample with 3% salt concentration had the highest protein concentration, by the second week, the three samples all had very close concentration, the protein concentration of the 3% sample had reduced while the sample with 1% concentration had increased. From the result the sample preserved by 2% salt had a higher protein concentration and maintained all through the storage period. In the control sample colour change and maggot infestation was noticed on the third day of storage.
Conclusion
This work concluded that to increase the shelf life of fermented African locust bean seeds, water must be totally eliminated from it. Total dryness of the fermented condiment will preserve the seed and increase its utilization especially in protein deficient food formulation. This will reduce scarcity (due to very short shelf life) and improve its availability all year round. Drying will also get rid of the undesirable smell which has made Ire unpopular in the urban region.
The application of many advanced food preservation methods such as canning, refrigeration and irradiation have greatly reduced because of the high cost in their operations. Moisture content reduction was found to be an efficient method for preservation.
References
- Porcher M (2005) Multilingual multiscript plant name database [en línea]. University of Melbourne. Institute for Land & Food Resources. Melbourne, Australia.
- Purcell LC, Salmeron M, Ashlock L (2014) Soybean growth and development. Arkansas Soybean Handbook. University of Arkansas, Fayetteville, USA.
- Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of earth’s nitrogen cycle. Science 330(6001): 192-196.
- Ojewumi ME, Omoleye JA, Ajayi AA (2016) Optimum fermentation temperature for the protein yield of parkia biglobosa seeds (Iyere), Proceeding of the 3rd International Conference on African Development Issues. pp. 584-587.
- Ojewumi ME (2016) Optimizing the conditions and processes for the production of protein nutrient from parkia biglobosa seeds. dissertation submitted to the Department of Chemical Engineering, Covenant University, Nigeria.
- Ojewumi ME, Omoleye JA, Ajayi AA (2016) The study of the effect of moisture content on the biochemical deterioration of stored fermented parkia biglobosa seeds. Open Journal of Engineering Research and Technology 1(1): 14-22.
- Ojewumi ME, Abiodun JO, Adesola AA (2016) The effect of different starter cultures on the protein content in fermented african locust bean (parkia biglobosa) seeds. International Journal of Engineering Research & Technology 5(4): 249-255.
- Kiers JL, Meijer JC, Nout MJ, Rombouts FM, Nabuurs MJ, et al. (2003) Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J Appl Microbiol 95(3): 545-552.
- Mital B, Garg S (1990) Tempeh technology and food value. Food Reviews International 6(2): 213-224.
- Hachmeister KA, Fung DY (1993) Tempeh: a mold-modified indigenous fermented food made from soybeans and/or cereal grains. Crit Rev Microbiol 19(3): 137-188.
- Reddy N, Pierson M (1994) Reduction in anti nutritional and toxic components in plant foods by fermentation. Food Research International 27(3): 281-290.
- Karyadi D, Lukito W (1996) Beneficial effects of tempeh in disease prevention and treatment. Nutrition reviews 54(11): S94.
- Kiers JL, Rombouts FM, Nout MJ (2000) In vitro digestibility of processed and fermented soya bean, cowpea and maize. Journal of the Science of Food and Agriculture 80(9): 1325-1331.
- Kiers J, Rombouts F, Nout M, Van Laeken AE (2000) In vitro digestibility of Bacillus fermented soya bean. Int J Food Microbiol 60(2-3): 163-169.
- Kiers J, Nout M, Rombouts F, Nabuurs M , vander MJ (2002) Inhibition of adhesion of enterotoxigenic Escherichia coli K88 by soya bean tempe. Lett Appl Microbiol 35(4): 311-315.
- Ojewumi ME, Ejemen VA, Taiwo OS (2018) A bioremediation study of raw and treated crude petroleum oil polluted soil with aspergillus niger and pseudomonas aeruginosa. Journal of Ecological Engineering 19(2): 226-235.
- Kalavi F, Muroki N, Omwega A (1996) Effect of tempe-yellow maize porridge and milk-yellow maize porridge on growth rate, diarrhoea and duration of rehabilitation of malnourished children. East African medical Journal 73(7): 427-431.
- Soenarto Y, Sudigbia I, Herman H, Karmini M, Karyadi D (2001) Antidiarrheal characteristics of tempe produced traditionally and industrially in children aged 6-24 months with acute diarrhea. Paediatrica Indonesiana 41(2): 88-95.
- Odunfa SA (1981) Microbiology and amino acid composition of Ogiri-a food condiment from fermented melon seeds. Molecular Nutrition & Food Research 25(9): 811-816.
- Omafuvbe B, Abiose S, Shonukan O (2002) Fermentation of soybean (Glycine max) for soy-daddawa production by starter cultures ofBacillus. Food Microbiology 19(6): 561-566.
- Ouoba L, Rechinger K, Barkholt V, Diawara B, Traore AS, et al. (2003) Degradation of proteins during the fermentation of African locust bean (Parkia biglobosa) by strains of Bacillus subtilis and Bacillus pumilus for production of Soumbala. J Appl Microbiol 94(3): 396-402.
- Ouoba LI, Diawara B, Amoa AWk, Traoré AS, Møller PL (2004) Genotyping of starter cultures of Bacillus subtilis and Bacillus pumilus for fermentation of African locust bean (Parkia biglobosa) to produce Soumbala .Int J Food Microbiol 90(2): 197-205.
- Ojewumi ME, Omoleye JA, Ajayi AA (2017) Optimization of fermentation conditions for the production of protein composition in parkia biglobosa seeds using response surface methodology. International Journal of Applied Engineering Research 12(22): 12852-12859.
- Ojewumi ME, Omoleye JA, Ayoola AA (2018) Effects of salting and drying on the deterioration rate of fermented parkia biglobosa seed. Journal of Nutritional Health & Food Engineering 8(1): 1-5.
- Ojewumi ME, Omoleye JA, Nyingifa AS (2018) Biological and chemical changes during the aerobic and anaerobic fermentation of African locust bean. International Journal of Chemistry Studies 2(2): 25-30.
- Kolapo A, Popoola T, Sanni MO, Afolabi RO (2007) Preservation of soybean daddawa condiment with dichloromethane extract of ginger. Research Journal of Microbiology 2(3): 254-259.
- Gernah D, Inyang C, Ezeora N (2007) Incubation and fermentation of African locust beans (Parkia Biglobosa) in production of “Dawadawa”. Journal of Food Processing and Preservation 31(2): 227-239.
- Ojewumi ME, Eluagwule B, Ayoola AA, Ogunbiyi AT, John A, et al. (2017) Termiticidal effects oAfrican locust bean (Parkia biglobosa) seed oil extracts. International Journal of Current Research 9(7): 53929-53934.
- Ojewumi ME, Okeniyi JO, Ikotun JO, Okeniyi ET, Ejemen VA, et al. (2018) Bioremediation: Data on pseudomonas aeruginosa effects on the bioremediation of crude oil polluted soil. Data in Brief 19: 101-113.
- Ojewumi ME, Adeeyo OA, Akingbade OM, Babatunde DE, Omodara OJ, et al. (2018) Evaluation of glucose syrup produced from cassava hydrolyzed with malted grains (rice, sorghum & maize).International Journal of Pharmaceutical Sciences and Research 9(8): 1000-1011.
- Ojewumi ME, Adeyemi AO, Ojewumi EO (2018) Oil extract from local leaves-an alternative to synthetic mosquito repellants, Pharmacophore 9(2): 1-6.
© 2018 Modupe Elizabeth Ojewumi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






