- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Phosphorus Adsorption a Foamed Waste Glass
Harada Hiroyuki*1, Asmak A Filiana1, Nishikawa Hanami1 and Tomoyuki Miyamoto2
1Purefectural University of Hitohima University, Japan
2COCCO LTD, Japan
*Corresponding author: Harada Hiroyuki, Purefectural University of Hitohima University, Japan
Submission: February 25, 2018;Published: May 11, 2018

ISSN 2640-9208 Volume2 Issue1
Introduction
Phosphorus ores will be exhausted in about 80 years [1]. The development of phosphorus-recycling systems would help to counteract phosphorus depletion. Foamed glass materials have a porous structure that incorporates a large number of voids and are conventionally used as civil engineering materials or aggregates for construction [2]. Since calcium carbonate is used as the foaming agent, and it can be used as an adsorbent for phosphorus. In this study, we investigated phosphorus removal from environmental water using foamed glass.
Materials and Experimental Methods
Batch experiment
The samples of foamed glass tested in this study were collected from a production site in Kure City, Hiroshima, Japan (Figure 1). A model environmental water sample with a phosphate concentration of 1mM was pre pared and its initial pH was adjusted from 2 to 7 with 0.1M sulfuric acid and sodium hydroxide solution. The solution (40mL) and the adsorbent (1g), i.e. a liquid/solid ratio of 20mL/g, were placed in a 50mL centrifuge tube in a small rotating agitator in a thermostatic chamber at 30°C. Agitation was performed at 40rpm for 24h. After separation, filtration was performed with 5 type C filter paper and the phosphate in the filtrate was determined by molybdenum blue spectrophotometers.
Figure 1: Formed glass.

Continuous treatment experiment
Foamed glass was into a pressure-resistant glass column of diameter 1cm and length 30cm, so that the glass was filled to 15cm from the top of the column. Raw water was supplied from the bottom to obtain treated water. A raw water sample with suspended solid concentration 18mg/L, pH 4.2, phosphate concentration 2.5mg/L and nitrate nitrogen concentration 1.0mg/L was obtained from a wastewater treatment facility at the Prefectural University of Hiroshima and used for this experiment.
Result and Discussion
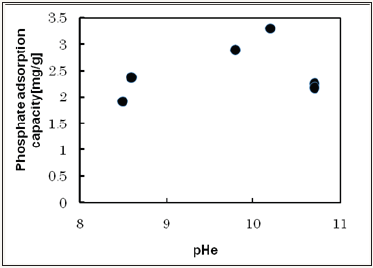
Figure 2 shows the effect of the equilibrium pH on the adsorption capacity. Initial pH adjusted pH 2-7 and they are shifted to 8.4-10.8 in equilibrium condition. Adsorption capacity shows 1.9mg/g~3.3mg/g. The saturated adsorption amount was estimated 3.3mg/g. The amount of material adsorbed per gram of adsorbent (q) was calculated from the difference between the concentrations before and after treatment,
Figure 2: The effect of the equilibrium pH on the adsorption capacity.
the treatment volume and the equilibrium concentration (Ce). The values of ln(q) and ln(Ce) can be give the Fruindrich relationship equation based on Figure 2.
Figure 3: Isothermal adsorption experiment (30oC, pHi4).
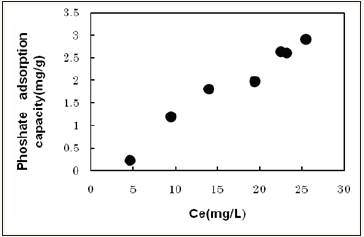
Q=0.032Ce1.43
R2=0.93
Figure 3 Batch experiment the concentration of treated water gradually decreased from 1.5 to 0.2mg/L, after that became constant at about 0.5 mg/L. No clear breakthrough was observed. This gradually decreases in concentration due to diffusion into the inside. After that, it is thought that the diffusion adsorption to the inside decreased and became flat (Figure 4).
Figure 4: Results of continuous experiment.

Acknowledgement
This research was funded by the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Grant no. 15K00605).
References
- Abelson PH (2015) Science. p. 283.
- Kazmina OV, Tokarev AY, Vereshchagin (2016) Resource-efficient technologies. 2: 23-29.
© 2018 Harada Hiroyuki. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






