- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
The Limitations of Food Intake and Biomarkers in the Prevention of Chronic Diseases
Martins IJ1,2,3*
1Edith Cowan University, Australia
2The University of Western Australia, Australia
3Hollywood Medical Centre, Australia
*Corresponding author: Dr. Martins IJ, Centre of Excellence in Alzheimer's Disease Research and Care School of Medical Sciences, Edith Cowan University, School of Psychiatry and Clinical Neurosciences, The University of Western Australia, McCusker Alzheimer's Research Foundation, 270 Joondalup Drive, Joondalup, Western Australia 6027, Australia
Submission: October 26, 2017; Published: November 13, 2017

ISSN 2640-9208 Volume1 Issue1
Keywords
Keywords: Dietary biomarkers; Plasma; Diagnosis; Central nervous system; Metabolic disease biomarkers; Sirtuin 1; Non alcoholic fatty liver disease; Species; Mitochondria; Agriculture; Diabetes; Nutrition
Editorial
The links between high fat/high sugar diets with insulin resistance and non alcoholic fatty liver disease (NAFLD) [1] has escalated with recent research that is relevant to the role of healthy diet consumption that may reverse NAFLD connected to Type 2 and Type 3 diabetes [2,3]. A low calorie diet is essential for the treatment of NAFLD and the benefit of this dietary regime is to activate the nuclear receptor Sirtuin 1 (Sirt 1) that is connected to improvements in glucose, cholesterol and antimicrobial activity [4]. Sirt 1 is a nicotinamide adenine dinucleotide (NAD+) dependent class III histone deacetylase that targets transcription factors to adapt gene expression to metabolic activity and its deacetylation of nuclear receptors indicates its critical role in insulin resistance in rodents, livestock and man [4]. High fibre diets [5] are essential to maintain glucose and cholesterol homeostasis with its maintenance of nuclear gene expression (Sirt 1) essential to prevent mitochondrial apoptosis and programmed cell death.
Major interests in food intake and the effects on various plasma biomarkers has been the focus of many research groups [6,7]. A review of existing literature on currently available dietary biomarkers include novel biomarkers of specific foods and dietary components that has revealed several dietary biomarkers in need of additional validation research [8-11]. The availability of biomarkers that determine the intake of specific foods and dietary components could greatly enhance nutritional research targeting with reversal of global disease such as NAFLD [12]. Identification of specific foods that induce changes in novel biomarkers such as Sirt 1 [13,14] are essential to determine the importance of existing literature with relationship to dietary foods and biomarkers. The projected cost of plasma and cell biomarker analysis is expected to cost by the year 2012 approximately 52 billion dollars, with relevance to major efforts to assess progression and severity of diseases with relevant these biomarkers are connected to dietary biomarkers that may delay the severity of progression of global chronic disease.
In previous research studies experiments in rodents and man [15,16] have indicated the role of various diets that may accelerate hepatic lipid metabolism with reversal of NAFLD. The progression and severity of NAFLD may not be delayed without the measurement of plasma Sirt 1 [13,14]. In rodents low calorie diets may increase hepatic lipid metabolism with increased plasma Sirt 1 levels [15]. In obese man and individuals with NAFLD levels of plasma Sirt 1 have been shown to be decreased [13,14] in spite of additional research on specific foods and dietary components with the measurement of several dietary biomarkers that may assist to prevent the progression of various chronic diseases [6-11].
Dietary interventions with the use of nutrigenomic diets are required early in life to reverse the defective adipose tissue-liver interaction that leads to NAFLD [17]. The role of nutrigenomic diets to activate the nuclear receptor Sirt 1 has become important with relevance to the nuclear-mitochondria interaction in the adipose tissue and liver cells. In rodents food restricted diets that do not contain various contaminants may allow nuclear Sirt 1 regulation of the immune system with mitochondrial biogenesis relevant to reversal of NAFLD [17]. Food quality has led to the identification of various contaminants in foods by microbiological spoilage associated with the inhibition of Sirt 1 with defective hepatic lipid metabolism and the induction of NAFLD.
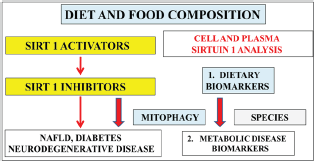
In the developing world nuclear Sirt 1 repression by bacterial lipopolysaccharides and the mycotoxin patulin (Figure 1) may not allow Sirt 1 release into the plasma and central nervous system with diseases linking to core body temperature defects related to diabetes and neurodegenerative diseases [18-20]. In the developed world various inhibitors of Sirt 1 (Figure 1) should be carefully assessed to stimulate Sirt l release into the blood plasma. Diagnostic tests that measure plasma analytes such as lipids, hormones, liver/ kidney function tests, drug levels, immune tests, enzymes and metals (zinc/magnesium) should be conducted with Sirt 1 and heat shock protein analysis to validate the importance of diagnostic plasma analysis in the diagnosis of global chronic disease.
Figure 1: Dietary interventions with the use of Sirt 1 activators are essential to reverse the effects of Sirt 1 inhibitors with relevance to diet and food composition. Defective adipose tissue-liver interaction that leads to NAFLD and global chronic disease involve mitophagy that require the analysis of plasma Sirt 1 with relevance to dietary and metabolic disease biomarkers. Relevance of cell and plasma Sirt 1 analysis is now important to chronic disease in man and various species.
Dietary biomarkers and dietary components that have stimulated the need for additional validation research now require the measurement of caffeine in the blood plasma. Caffeine is a Sirt 1 modulator and its presence as a food additive, plant component and in coffee requires its measurement with relevance to effects on Sirt 1 release in the blood plasma and CNS. The importance of plasma Sirt 1 and its relevance to plasma lipoprotein metabolism [15,21] may indicate its critical role in the beneficial properties of olive oil [22]. In agriculture and the livestock industry the role of dietary caffeine with various diets/dietary components need to be reassessed with relevance to the release of Sirt 1 from the liver and brain cells in various species that may be associated with chronic diseases in these animals. Activators of Sirt 1 (Figure 1) as a food additive [5] may be essential in various diets in livestock and man to maintain glucose, cholesterol and anti-microbial activity [2,4,21] related to programmed cell death and organ disease.
Conclusion
Extensive literature on dietary plasma biomarkers that include novel biomarkers of specific foods and dietary components have been assessed with relevance to various global chronic diseases. The connections between dietary plasma biomarkers and metabolism now require the plasma analysis of Sirt 1. In agriculture and the livestock industry healthy diets with relevance to Sirt 1 (cells/ plasma) and dietary biomarkers have become important with relevance to mitochondrial biogenesis and regulation of metabolism in various species. Sirt 1 activators in the diet are critical to prevent the role of various Sirt 1 inhibitors with defective nuclear- mitochondria interactions related to programmed cell death and chronic disease. Dietary plasma biomarkers need to be analysed with metabolic disease biomarkers to determine the primary role of dietary biomarkers in global chronic disease outcomes.
Acknowledgement
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer's Research Foundation and the National Health and Medical Research Council.
References
- Ragab SM, Abd Elghaffar SKH, El-Metwally TH, Badr G, Mahmoud MH, et al. (2015) Effect of a high fat, high sucrose diet on the promotion of nonalcoholic fatty liver disease in male rats: the ameliorative role of three natural compounds. Lipids Health Dis 14: 83.
- Martins IJ (2017) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. J Diabetes Metab Disord 4: 019.
- Martins IJ (2015) Diabetes and organ dysfunction in the developing and developed. Global Journal of Medical Research: F Diseases 15(1): 15-21.
- Martins IJ (2017) Antimicrobial activity inactivation and toxic immune reactions induce Epilepsy in human. J Med Discov 2: 1-7.
- Martins IJ, Fernando WMABD (2014) High fibre diets and Alzheimer's disease. Food and Nutrition Sciences 5(4): 410-424.
- Savolainen O, Lind MV, Bergstrom G, Fagerberg B, Sandberg AS, et al. (2017) Biomarkers of food intake and nutrient status are associated with glucose tolerance status and development of type 2 diabetes in older Swedish women. Am J Clin Nutr.
- Dencker M, Gárdinger Y, Bjorgell O, Hlebowicz J (2017) Effect of food intake on 92 biomarkers for cardiovascular disease. PLoS One 12(6): e0178656.
- Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, et al. (2012) Dietary biomarkers: advances, limitations and future directions. Nutr J 11:109.
- Gibbons H, Michielsen CJR, Rundle M, Frost G, McNulty BA, et al. (2017) Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol Nutr Food Res 61(10).
- Catalán Ú, Rodríguez MÁ, Ras MR, Maciá A, Mallol R, et al. (2013) Biomarkers of food intake and metabolite differences between plasma and red blood cell matrices; a human metabolomic profile approach. Mol Biosyst 9(6): 1411-1422.
- O'Gorman A, Gibbons H, Brennan L (2013) Metabolomics in the identification of biomarkers of dietary intake. Comput Struct Biotechnol J 4: e201301004.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. (2016) Global epidemiology of non-alcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1): 73-84.
- Martins IJ (2017) The Future of biomarkers tests and genomic medicine in global organ disease. Arch Infect Dis Ther 1: 1-6.
- Martins IJ (2017) Biomarker tests and ageing science. Ageing Sci Ment Health Stud 1: 1-2.
- Martins IJ (2014) Induction of NAFLD with increased risk of obesity and chronic diseases in developed countries. Open Journal of Endocrine and Metabolic Diseases 4(4): 90-110.
- Martins IJ, Redgrave TG (2004) Obesity and post-prandial lipid metabolism. Feast or Famine? J Nutr Biochem 15(3): 130-141.
- Martins IJ (2015) Unhealthy nutrigenomic diets accelerate NAFLD and adiposity in global communities. Journal of molecular and Genetic medicine 9(1): 1-11.
- Martins IJ (2017) Dietary interventions reverse insulin and synaptic plasticity defects linking to diabetes and neurodegenerative diseases. SL Nutrition and Metabolism 1(111): 1-5.
- Martins IJ (2017) The future of genomic medicine involves the maintenance of sirtuin 1 in global populations. Int J Mol Biol 2(1): 00013.
- Martins IJ (2017) Regulation of Core Body Temperature and the Immune System Determines Species Longevity. Curr Updates Gerontol 1: 1-6.
- Martins IJ (2017) Caffeine with Links to NAFLD and accelerated brain aging. Non-alcoholic fatty liver disease-molecular bases, prevention and treatment. In Tech-Open Science Open Minds | In Tech Open In Press.
- Martins IJ (2017) Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet 3: 1-8.
© 2017 Martins IJ. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






