- Submissions

Full Text
Novel Research in Sciences
Selenium Deprivation Alters Cytoskeletal Structure in Human Umbilical Vein Endothelial Cells (HUVECS)
Adrian M Taranto1, Jalen D Hurt1, David A Bradford1, Jaden Hines1, Brendon Davis2, Alexcia Smith2, Kaela Battles3, Sequan Patterson4, Felix Adusei-Danso1 and Emmanuel D Williams1*
1Livingstone College, Department of Biology, Salisbury, North Carolina, US
2Southern University and A&M College, Baton Rouge, Louisiana, US
3Xavier University School of Pharmacy, New Orleans, Louisiana, US
4Appalachian State University, Boone, North Carolina, US
*Corresponding author:Emmanuel D Williams, Livingstone College, Department of Biology, Salisbury, North Carolina, US
Submission: February 15, 2023;Published: March 20, 2023
.jpg)
Volume14 Issue2March , 2023
Abstract
including genetics, lifestyle, and nutrition. Nutrient signaling has been shown to modulate the onset, progression, and/or delay of many age-associated diseases including cardiovascular disease and neurodegenerative decline. While glycemic and lipid flux have been linked to the premature appearance of in vitro biomarkers of aging, the role of trace metals in age-associated cellular changes remain less clear. The trace metal Selenium (Se) is an essential trace mineral that supports many bodily processes including free radical scavenging, thyroid function, and maintenance of cognitive ability. Our research focused on the potential cellular changes in Human Umbilical Vascular Endothelial Cells (HUVECs) in response to Se deprivation. Exposed to a 48-hour continuous reduced Se exposure, we observed a reduced f-actin protein and gene expression. Interestingly, we observed a compensatory increase in the intermediate filament vimentin, which suggest that Se may have an important role in cytoskeletal maintenance and rearrangement.
Keywords:Selenium; Endothelial; Oxidative stress; Vimentin; Actin; Nutrients; Aging
Introduction
The process of aging has long fascinated the scientific community. In the last thirty years, the impact of nutrition and metabolism on organismal aging has been established. Clinical manifestations of nutrient flux are well-implicated in models of health and disease such as Cardiovascular Disease and stroke (CVD), diabetes, and obesity [1-3]. In vitro and in vivo studies assessing nutrient flux also seem to mimic clinical outcomes: aberrant nutritional levels drive the premature appearance of biomarkers of aging [4]. Diets high is fats have been shown to accelerate biomarkers of aging in murine kidney models, including increased oxidative stress, impaired fatty acid oxidation, and increased DNA methylation patterns associated with Senescence-Associated Heterochromatin Foci (SAHF) [5]. Further studies have linked increased consumption of low-density lipoproteins with the increased expression and secretion of proinflammatory cytokines and increased DNA methylation patterns [6-9]. Extremely low levels of plasma low-density proteins in younger populations, although rare, have been associated with age-associated diseases including cancer and neurodegenerative diseases [10]. Glycemic flux has also been linked to the aging. Clinical hyperglycemia led to insulin resistance and type- 2 diabetes, a “gateway” to other age-associated outcomes. In vitro analysis of hyperglycemia in fibroblast and endothelial cell models have shown metabolically active cells void of replicative ability, increased secretion of pro-inflammatory cytokines, increased oxidative stress [11,12]. Previous in vitro studies from our research group show that similar to elevated glucose stress, glucose deprivation also led to reduced Population Doubling Levels (PDLs) in pooled young endothelial cells [13]. Further mechanistic studies in rat cardiomyocytes (H9C2) cells, also elucidate reduced mitochondrial membrane potential, altered mitochondrial repair, and respiratory uncoupling (oxidative stress) in response to glucose deprivation [14].
While our understanding of macromolecules and their importance to aging continues to develop rapidly, less is clear about the role (s) of trace elements of the preservation and/or acceleration of aging. Trace elements are inorganic elements or compounds present in living tissues in very small quantities. Some of them are known to be nutritionally essential while others while the remainder are considered to be nonessential [15]. Trace elements function in various roles including as (1) catalysts in enzyme complexes; (2) participants in oxidation-reduction reactions in energy metabolism; and (3) in cellular transport (i.e., iron functions in the transport of oxygen via hemoglobin and myoglobin [16-19]. Optimal levels of trace elements are essential to physiological homeostasis. Excessive levels of any trace elements are toxic. For example, excess plasma levels of iron (hemochromatosis) are associated with pancreatic, cardiac, hepatic, and neurological dysfunction primarily by production of free radicals [20-23]. Along with high levels of other trace elements and the concomitant oxidative stress, it is not surprising that excess levels these metals are associated with the accelerated appearance of biomarkers of aging. In fact, this is the basis of the Free Radical Theory of Aging. However, what remains less clear is the role of trace metal deprivation on aging. Our research sought to characterize trace metal deprivation on cellular aging. The trace metal Se was chosen for its initial characterization because of its fundamental importance to human health, particularly with beneficial. As an integral part of selenoproteins, Se has structural and enzymic roles, including functioning as an antioxidant and catalyst for the production of active thyroid hormone [24]. In addition, Se deficiency has been linked to adverse mood states, implicating that it may have a role neurological function. Findings have linked Se to cardiovascular disease risk although other conditions involving oxidative stress and inflammation have shown benefits of a higher Se status [25]. Collectively, the role of Se may have direct and indirect effects on aging. Human endothelial cells present an excellent cellular model to study nutrient stress in vitro. Endothelial cells form the inner lining of a blood vessel and provides a selective barrier between the vessel wall and blood [26]. Because of its direct contact with blood, endothelial cells are sensitive to and in response to nutritional flux. The endothelial cell reacts with physical and chemical stimuli within the circulation and regulates hemostasis, vasomotor tone, and immune and inflammatory responses [27-30]. To characterize the role of Se deprivation on cellular aging, we cultured Human Umbilical Vein Endothelial Cells (HUVECs) in the presence of Se rich vs. Se-deficient media and monitored cellular protein expression, gene expression, and cytoskeletal rearrangements. We found that Se deprivation reduces the appearance and expression of the cytoskeletal protein f-actin while up regulating the intermediate filament vimentin in early passage cells. Se deprivation decreased the overall cells size and slowed the population doubling rate in younger passage cells. Collectively, our findings show the Se levels, even in younger more fit cells, accelerates the premature appearance of biomarkers of aging.
Material and Materials
Cell culture
Figure 1: Schematic depiction of trace metal (Se) deprivation in HUVECs cells were cultured in full complement media for 24 hours, after which they were passaged in normal OR Se-deficient media. After which RNA and protein was harvested for PCR and Immunoblot Analysis. Cells were also fixed for microscopic analysis of cell structure.
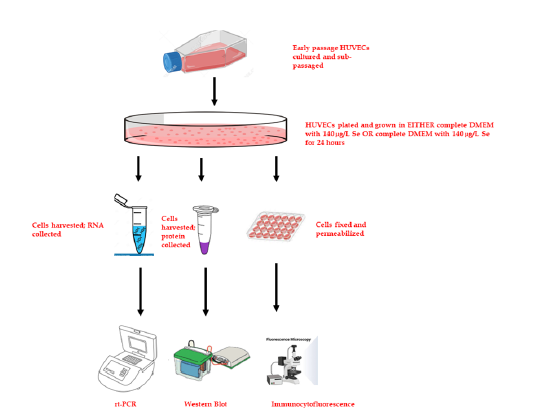
Cell cultures were prepared and maintained according to standard cell culture procedures. Commercially available pooled Human Umbilical Vein Endothelial Cells (HUVEC; Lonza, Basel, Switzerland) were cultured in Dubelco’s Minimal Essential Media (DMEM) with no glucose supplemented with 1μg/ml hydrocortisone, 50μg/ml gentamicin, 50ng/ml amphotericin-B, 10ng/ml epidermal growth factor and Human Platelet Lysate (HPL). Formulation of Se-free media is difficult due to the uncertain levels of Se in basal media-supplemented serum (i.e., Fetal Bovine Serum; FBS). As a precaution, our studies will omit the use of serums and will instead use HPL. HPL contains comparable growth factors as FBS, including insulin-like growth factor, epidermal growth factor. 100mg/dL of glucose was supplemented and vitamins and trace metals were added or withheld (Se) according to desired level with 140μg/L serving a physiological reference and 50μg/L serving as “reduced” level (Figure 1). HUVECs were incubated and cultured in 5% CO2 and 95% air at 37ᵒ C till the tenth passage of cell generation. Cells were passaged at regular intervals depending on their growth characteristics using 25% trypsin and re-plated.
Western blot analysis
HUVECs were harvested and lysed in RIPA lysis buffer containing 10mM protease inhibitors (phenylmethylsulfonyl fluoride, PMSF, Beyotime, China). A BCA protein assay kit (Beyotime) was used to determine the protein concentrations. Then, loading buffer (Beyotime) was added to the samples. The cellular proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, and the separated proteins were electrically transferred onto cellulose membranes (Millipore, USA). Then, the membranes were blocked with 5% nonfat dry milk in TBST (20mM Tris-HCl pH 7.4, 150mM NaCl, and 0.05% Tween-20) for 1h. The membranes were washed with TBST and probed with primary antibodies overnight at 4 °C. The bound primary antibodies (F-actin (C-2)-Santa Cruz, USA; Vimentin (V9)-Santa Cruz, USA; GAPDH (0411)-Santa Cruz, USA; and α-tubulin (TU-02)-Santa Cruz, USA) were detected with secondary antibodies conjugated with horseradish peroxidase (HRP, Abcam, UK) and visualized by enhanced chemiluminescence (Millipore). Cyclophilin-A and α-actin were used as an internal reference.
Quantitative real-time PCR
Total RNA was extracted from cultured HUVECs using Trizol
solution (In vitro gen) and reverse transcribed into cDNA with a
Reverse Transcriptase kit (Invitrogen) according to manufacturer’s
instructions. The concentration and purity of RNA were measured
using a NanoDrop 2000 spectrophotometer (Thermo, USA). The
expression of f-actin and vimentin were detected using a TaqMan
mRNA assay kit (Biosystems). The relative expression was
determined by the 2-ΔΔCt method with GAPDH as the internal
reference. Samples were analyzed in triplicate. The primer
sequences used were the following.
F-actin-F: CACCATTGGCAATGAGCGGTTC,
f-actin-R: AGGTCTTTGCGGATGTCCACGT,
Vimentin-F: AGGCAAAGCAGGAGTCCACTGA,
Vimentin-R: ATCTGGCGTTCCAGGGACTCAT,
GAPDH-F: GGAGCGAGATCCCTCCAAAAT,
GAPDH-R: GGCTGTTGTCATACTTCTCATGG.
qRT-PCR was performed in 96-well plates using the Light Cycler 480 system (Roche, Swiss). Each 20-μL reaction contained 10μL of TransStart Green qPCR SuperMix (Transgen Biotech, China), 0.5μL of each primer (10μmmol/L), 8μL of ddH2O, and 1μL of cDNA. PCR conditions were as follows: 95 °C for 1min, followed by 40 cycles at 95 °C for 20s, and 61 °C for 31s.
Immunocytochemistry
HUVEcs were fixed in 4% paraformaldehyde for 20 minutes subsequently washed in PBS. HUVECs were immersed in 0.2% (v/v) Triton X-100 for 7 minutes at room temperature to permeabilize cells, then blocked in 1mL of 3% (v/v) BSA and 0.2% Triton X-100 in PBS at 4 °C overnight on a rocking platform shaker. Primary and secondary antibodies were diluted in 3% BSA and 0.2% Triton X-100. Anti-(F-actin (C-2)-Santa Cruz, USA; Vimentin (V9)-Santa Cruz, USA; GAPDH (0411)-Santa Cruz, USA; and α-tubulin (TU-02)- Santa Cruz, USA) was used at 1:500 and incubated at 4 °C overnight on a rocking platform shaker. Samples were washed three times with 3% (v/v) BSA in 0.2% (v/v) Triton X-100 before the addition of fluorophore-labeled secondary antibodies (AlexaFluor series, Life Technologies) directed against the appropriate host. Then 1mL of secondary antibodies diluted 1:500 was added to each construct, and samples were incubated at 4 °C overnight on a rocking platform shaker. Samples were then washed with 3% (v/v) BSA in 0.2% (v/v) Triton X100 at least three times before the addition of DAPI (5μg/ mL) in PBS. All immunofluorescence images were captured using a Nikon A1-Rsi fluorescence microscope. Images were obtained with a Zeiss Axio Imager Z2 microscope.
Cell size analysis
All image analysis (cell segmentation, cell size, and measurements of fluorescence intensity and nuclear size) and data analysis was performed with custom-written tools in Nikon-XD. The mean and variance of cell size as a function of age were calculated by nonparametric regression, as described by Wasserman [31]. To quantify the influence of Se on the variation in cell size, we used the normalized median absolute deviation (MAD), that is MAD(x) median(x).
Results
Se deprivation reduces f-actin gene and protein expression; vimentin expression increases
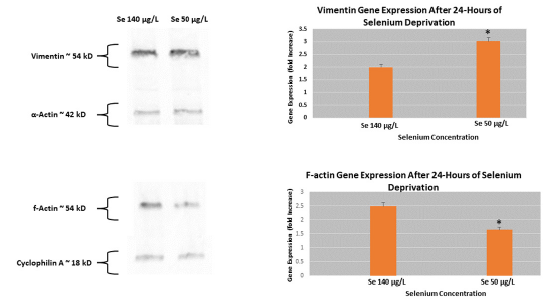
Actin is one of the most abundant cellular proteins and is in constant flux based on the needs of the cell. Exposure of HUVECs to normal (140μg/L) to reduced (50μg/L) for 24 hours showed a decrease in f-actin gene and protein expression (Figure 2). More specifically, we observed a 32% reduction in f-actin gene expression in cells deprived of Se compared to cells supplemented with physiological normal levels of the trace metal. We next proposed the hypothesis of a compensatory mechanism in which other micro-, intermediate, and/or macro-filaments (microtubules) are upregulated in response to reduced f-actin levels. An initial gene expression analysis of α-actin, keratin, vimentin, and tubulin were performed; only vimentin showed a comparable change in gene expression. A confirmatory PCR analysis showed a 42% increase in vimentin expression in response to reduced Se exposure. Western blot analysis of both vimentin supported gene expression levels; vimentin protein expression increased 44% and f-actin protein expression levels were decreased 54% in HUVECs when subjected to 24-hour Se deprivation.
Figure 2: Cytoskeletal gene and protein expression in HUVECs in response Se deprivation Microfilament (f-actin) and Intermediate filament (vimentin) were measured in response to Se deprivation. Se deprivation in HUVECs showed an increased vimentin gene and protein expression level while a decrease in f-actin gene and protein levels was observed compared to Se-rich exposure. *p≤0.05.
Se deprivation alters cell morphology and reduces overall endothelial cell size
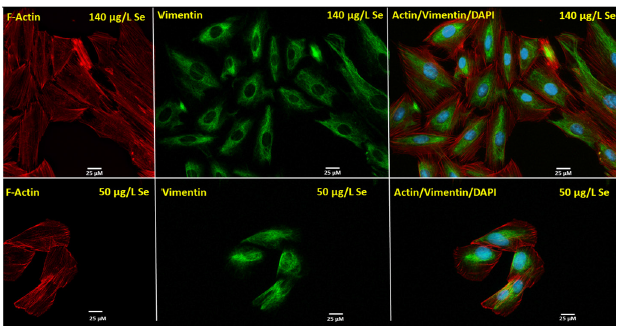
Observations in differential protein and gene expression provoked investigation to cell morphological changes in response to Se deprivation. In addition to f-actin and vimentin, our studies aimed at characterizing overall cell size in response to Se deprivation. Exposure of HUVECs to reduced Se levels showed an altered F-actin distribution in which filaments appeared to localize at the periphery of the cell (Figure 3). This phenomenon appears to be a signature of endothelial cell stress: the formation of actin stress fibers. Interestingly, immunocytoflourescence staining showed cellular rearrangement of vimentin, one in which the intermediate filament re-localized to more of a perinuclear/central localization in response to Se deprivation compared to a definitive cytoplasmic localization under normal Se conditions.
Figure 3:Altered cell morphology in HUVECs in response se deprivation Microfilament (f-actin) and Intermediate filament (vimentin) were assessed via immune cytoflourescence in response to Se deprivation. Se deprivation in HUVECs showed a morphometric rearrangement of f-actin to the periphery of the cells compared to that of HUVECs cultured in physiologically normal Se levels. Vimentin distribution also changed in response to Se deprivation, becoming more perinuclear.
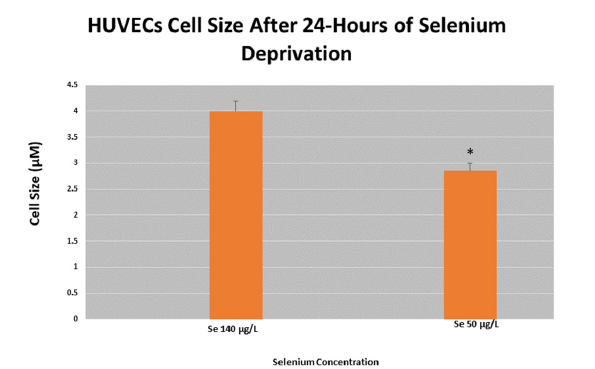
Previous studies have implicated cell size with altered cell function [32]. Concomitant with altered cytoskeletal rearrangements, we next sought to investigate if the overall cell size changed. Using a morphometric analysis with light microscopy and counterintuitive to our hypothesis, exposure of HUVECs to a 24-hour reduced Se stress reduced the overall size (Figure 4). More specifically, cell size was reduced 38% in response to reduced Se levels.
Figure 4:Analysis of cell size in HUVECs in response Se deprivation Ten (10) random fields in HUVECs were captured, normalized, and measured for cell size in response to Se deprivation. Se deprivation in HUVECs showed an overall reduction in cell size compared to HUVECs supplemented with normal levels of Se. *p≤0.05.
Discussion
The role of trace metals in health and disease continues to be an intense area of research. The molecular mechanism(s) associated with trace metal deprivation have been sparsely characterized but may hold important roles in processes such as cancer and cellular aging. To begin our study on the potential cellular role, we held to the unifying theme that structure in large part determines function. Our report is the first known in vitro characterization on the role of Se in cytoskeletal integrity of endothelial cells. Our findings appear consistent with clinical outcomes reported with Se deficiency: reduced Se levels are associated with outcomes depended on altered cell morphology including extravasation, invasion, and metastasis of malignant cells. The altered f-actin dynamics observed appear to confirm the essential nature of Se. Our findings at the gene, protein, and morphological level stop short of establishing Se-linked reduction in f-actin. Rather, analysis of globular (g) actin appear to have no difference in gene and expression (data not shown) regardless of Se level. This appears to suggest that Se may be a direct component in the polymerization of f-actin and that in its reduced state, g-actin remains abundant. Reduced f-actin also poses an indirect role of Se: metabolism. ATP is needed to” charge” g-actin monomers before being added to the (+) end of elongating actin fibers (actin polymerization). Understanding Se’s role in oxidative stress as an antioxidant and the mitochondria’s role as a major ROS/RNS producer, it is plausible that a consequence of Se deprivation is one of mitochondrial damage and subsequent reduced mitochondrial capacity (ATP). No known studies have explored the role(s) of Se on cellular metabolism but is an area of interest in our future studies. F-actin redistribution to the cell periphery has been previously characterized in models of cellular stress and transformation, including tumorigenesis and metastasis. Known as “stress fibers” are contractile actomyosin bundles found in many cultured non-muscle cells, where they also have a central role in cell adhesion and morphogenesis. Focal-adhesion-anchored stress fibers also have an important role in mechanotransduction. In animal tissues, stress fibers are especially abundant in endothelial cells, myofibroblasts and epithelial cells. Our finding that intermediate filaments are upregulated as microfilaments are redistributed is the first report in endothelial cells. Moreover, it raises the question of whether the observed intermediate filament dynamics are the direct result of Se levels or rather a compensatory mechanism. Future studies into Se-intermediate dynamics are further needed and could elucidate may cellular processes that are associated with increased intermediate filaments including cellular senescence. Lastly, reduced cell size observed with Se deprivation is a phenomenon that needs further investigation but implicates a potential role for Se in another cytoskeletal entity: microtubules. Basic cellular processes including flux across membranes are contingent on cell size. As a result, changes in cell volume or surface area will have profound effects on metabolic flux, biosynthetic capacity, and nutrient exchange. In addition, the basic machinery of cell division in eukaryotes relies on microtubules, both to form the mitotic spindle and position it properly relative to the cortex. Because of the dynamic properties of microtubules, they are able to probe a limited range of lengths, and if cells get too big or too small, the mitotic apparatus may have difficulty working.
Acknowledgement
The reported work is the product of the generous biomedical philanthropy to Dr.(s) Thomas and Katheryn Williams.
References
- Packer M (2020) Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: Implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol 31(5): 907-919.
- Sang L, Ju HQ, Yang Z, Ge Q, Zhang Z, et al. (2021) Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat Metab 3(1): 90-106.
- Magaway C, Kim E, Jacinto E (2019) Targeting mTOR and metabolism in cancer: lessons and innovations. Cells 8(12): 1584.
- Rogers SC, Zhang X, Azhar G, Luo S, Wei JY (2013) Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J Gerontol Biol Sci Med Sci 68(12): 1469-81.
- Zheng X, Feng M, Wan J, Shi Y, Xie X, et al. (2021) Anti-damage effect of theaflavin-3'-gallate from black tea on UVB-irradiated HaCaT cells by photoprotection and maintaining cell homeostasis. J Photochem Photobiol B 224: 112304.
- Ma J, Yang X, Chen X (2021) C/EBPβ is a key transcription factor of ox-LDL inducing THP-1 cells to release multiple pro-inflammatory cytokines. Inflamm Res 70(10-12): 1191-1199.
- Li Y, Liu S, Wang YT, Min H, Adi D, et al (2020) TBL2 methylation is associated with hyper-low-density lipoprotein cholesterolemia: A case-control study. Lipids Health Dis 19(1): 186.
- Reeskamp LF, Venema A, Pereira JPB, Levin E, Nieuwdorp M, et al. (2020) Differential DNA methylation in familial hypercholesterolemia. EBioMedicine 61:103079.
- Chang PY, Pai JH, Lai YS, Lu SC (2019) Electronegative LDL from rabbits fed with atherogenic diet is highly proinflammatory. Mediators Inflamm 2019: 6163130.
- Zhang X, Liu J, Wang M, Qi Y, Sun J, et al. (2018) Twenty-year epidemiologic study on LDL-C levels in relation to the risks of atherosclerotic event, hemorrhagic stroke, and cancer death among young and middle-aged population in China. J Clin Lipidol 12(5): 1179-1189.
- Liu Y, Liu Y, He W, Mu X, Wu X, et al. (2022) Fibroblasts: immunomodulatory factors in refractory diabetic wound healing. Front Immunol 13: 918223.
- Clyne AM (2021) Endothelial response to glucose: Dysfunction, metabolism, and transport. Biochem Soc Trans 49(1): 313-325.
- Aberdeen H, Battles K, Taylor A, Garner-Donald J, Davis-Wilson A, et al. (2021) The aging vasculature: Glucose tolerance, hypoglycemia and the role of the serum response factor. J Cardiovasc Dev Dis 8(5): 58.
- Ma T, Huang X, Zheng H, Huang G, Li W, et al. (2021) SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev 2021: 9265016.
- Stojsavljević A, Perović M, Nešić A, Miković Ž, Manojlović D (2022) Levels of non-essential trace metals and their impact on placental health: a review. Environ Sci Pollut Res Int 29(29): 43662-43674.
- Kim SS, Meeker JD, Keil AP, Aung MT, Bommarito PA, et al. (2017) Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ Res 179: 108854.
- Wang WX, Tan QG (2019) Applications of dynamic models in predicting the bioaccumulation, transport and toxicity of trace metals in aquatic organisms. Environ Pollut 252: 1561-1573.
- Nishito Y, Kambe T (2018) Absorption mechanisms of iron, copper, and zinc: An overview. J Nutr Sci Vitaminol 64(1): 1-7.
- Shelby ML, Wildman A, Hayes D, Mara MW, Lestrange PJ, et al. ( 2021) Interplays of electron and nuclear motions along CO dissociation trajectory in myoglobin revealed by ultrafast X-rays and quantum dynamics calculations. Proc Natl Acad Sci USA 118(14).
- Porter JL, Rawla P (2022) Hemochromatosis. In: StatPearls [Internet] Treasure Island (FL): Stat Pearls Publishing, US.
- Kane SF, Roberts C, Paulus R (2021) Hereditary hemochromatosis: Rapid evidence review. Am Fam Physician 104(3): 263-270.
- Mehta KJ, Farnaud SJ, Sharp PA (2019) Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol 25(5): 521-538.
- Lal A (2020) Iron in health and disease: An update. Indian J Pediatr 87(1): 58-65.
- Kiełczykowska M, Kocot J, Paździor M, Musik I (2018) Selenium-a fascinating antioxidant of protective properties. Adv Clin Exp Med 27(2): 245-255.
- Gać P, Czerwińska K, Macek P, Jaremków A, Mazur G, et al. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ Toxicol Pharmacol 82: 103553.
- Dalal PJ, Muller WA, Sullivan DP (2020) Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol 190(3): 535-542.
- Gimbrone MA, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4): 620-636.
- Krüger-Genge A, Blocki A, Franke RP, Jung F (2019) Vascular endothelial cell biology: An update. Int J Mol Sci. 20(18): 4411.
- Goldenberg NM, Kuebler WM (2015) Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Compr Physiol 5(2):531-559.
- Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7(10): 803-815.
- Almeida AE, Stefani CM, Nascimento JA, Almeida NM, Santos AC, et al. (2014) An equation for the prediction of oxygen consumption in a Brazilian population. Arq Bras Cardiol 103(4): 299-307.
- Davie E, Petersen J (2012) Environmental control of cell size at division. Curr Opin Cell Biol 24(6): 838-844.
© 2023 Emmanuel D Williams. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






