- Submissions

Full Text
Novel Research in Sciences
Effect of Feeding System on Goat Cheese Quality
Galina MA1*, Higuera Piedrahita RI1, Dela Cruz H1, Olmos J2, Vazquez P3 and Sanchez N4
1Faculty of Higher Studies Cuautitlán, National Autonomous University of Mexico, Mexico
2Faculty of Veterinary Medicine and Zootechnics Autonomous University of Querétaro, Querétaro, Mexico
3Center for Research in Applied Science and Advanced Technology National Polytechnic Institute, Querétaro, Mexico
4University of Colima, Mexico
*Corresponding author:Miguel Ángel Galina, Faculty of Higher Studies Cuautitlán, Cuautitlan, Mexico
Submission: December 21, 2022;Published: January 09, 2023
.jpg)
Volume13 Issue1January , 2023
Abstract
The study was performed to compare milk quality on goat cheese managed under different feeding systems: exclusive grazing (EG), supplemented grazing (SG) or full confinement (FC). Sampling was obtained in 2020 and 2021 on 1418 dairy goats from Querétaro, Michoacán, Guanajuato and San Luis Potosí in Mexico. Among the 84 farms included in the trial, 25.3% were in FC (359 animals) fed corn silage, alfalfa hay, and commercial balanced concentrate (18% CP; 500 to 1000g/ head/day); in both extensive (EG) and supplemented (SG) exclusive grazing, vegetation consumed was a mixture of grasses; Bouteloua curtipendula, chloris virgata, bothriochloa saccharoides, leptochloa dubia, rhyncheltythurum roseum, panicum obtusum, bouteluoa repens, aristida adscensionis, setaria parviflora, urochloa fasciulata, pennisetum ciliare., legumes: prosopis leavigata, acacia farnesiana, acacia schaffneri, mimosa biuncifera., shrubs: celtis pallida, psilactis brevilingulata, jatropha dioica, zalazania augusta var. Augusta, verbasina serrata and cactus: opuntia affasiacantha, o. Amyctaea, o. Cretochaeta, o. Hytiacantha, o. Robusta, O. streptacanta, O. tomentosa and O. imbricata Group EG (30%, 426 does) were permanently on rangeland while SG (44.6%, 632 heads) was partially on rangeland supplemented with a commercial balanced feed. Average daily milk yield was significantly (P<0.05) different among groups: 1.6±.212kg (FC), 0.950±.272kg (SG) and 0.720±.153kg (EG). Feeding system also affected milk fatty acid profile, particularly the ω6/ω3 ratio: increasing the amount of concentrate in the diet significantly (P<0.05) improved ω6 or decreased ω3 concentration, together with a rise on antioxidants (polyphenols) under grazing and diminishing when were kept in full confinement. Level of alpha-tocopherol was higher (p<0.05) in grazing goats (193.02μg/100g), compared to SG: 119.83μg/100g and FC: 87.16μg/100g. The degree of antioxidant protection (DAP) increased significantly between treatments, with the highest value being observed in EG (13.2), followed by SG (7.3) and FC (4.1). It is concluded that there is an effect of the feeding system that directly influences the profile of fatty acids and the degree of antioxidant protection in cheese, being greater in milk that comes from grazing goats diminishing beneficial effects for human health. Cheese improved facilities due to farmer incomer, did not significantly affected quality, feeding system seemed to be the key component in the heathy structure of the product.
Keywords: Milk; Grazing; Pufa; Omega Fatty Acids; Antioxidants
Introduction
In recent years, the consumers’ demand for foods with high nutritional value has strongly increased [1]. Concerning those of animal origin, it is accepted that animal diet can affect food quality [2-6] in particular, nutrients produced by grazing ruminants are recognized by nearly all consumers, and farmers themselves, as functional foods [6,7]. On the other hand, dairy specialization and intensive farming have brought about an increase in the use of concentrates, thus reducing or even eliminating shrubland as a feed source in many countries [8]. Nevertheless, in some areas, there is still an abundant supply of natural grasses and leguminous trees, that allows to feed goats in rangeland, thus producing high quality milk [9].
However, previous studies on the nutritional quality of milk in Mexico demonstrated several benefits of grazing goats [9]. In fact, lower content of saturated fatty acids (SFA) and higher levels of ω3 polyunsaturated fatty acids (PUFA), were noted in milk from grazing animals compared to that from animals in full confinement [6,10,11]. It has been proven that a lower content of SFA favors human health [5,6], as well as it has been shown that ω3 PUFAs, arachidonic acid and docosahexaenoic acid (DHA), are able to improve oxidative stress. This is critical, since oxidative stress is characterized by a reduction in the capacity of the endogenous system to act against oxidative attack directed to biomolecules, associated with different severe pathologies, such as cancer, cardiovascular diseases, type 2 diabetes, hypertension, and neurodegenerative diseases [6,10,11].
Other research focused on the importance of ω6/ω3 ratio [12], suggesting a value of 4:1 may prevent cardiovascular diseases up to a 70% reduction in mortality. More recently, the importance of maintaining a ω6/ω3 ratio lower than 4:1 was underlined [13,14] also because, in modern diets it results higher than 10 [15,16]. Thus, milk from grazing ruminants could have beneficial effects of human health, contributing to decrease the ω6/ω3 ratio of the diet and diminishing oxidative stress The objective of the present study was to evaluate, over two years, the effect of grazing on the milk fatty acids profile, particularly the ω6/ω3 ratio, and presence indirectly polyphenols content by the degree of antioxidant protection (DAP) by comparing 1418 lactating goats undergoing three different feeding systems in Mexico.
Materials and Methods
Experimental design and treatments
The experiment was performed on bulk milk of 84 farms (total of 1418 dairy goats) in Mexico, for two years: 2020 and 2022. The farms were in Querétaro (Latitude: 20.5931, Length: -100.392 20°, at 1820msnm), Michoacan (100°04’ north latitude, 93°15’ length, 1750 meters over the sea), Guanajuato (latitude 21.01, lenght-101.15′, over 550msnm), and San Luis Potosí (22° 36´ latitude, 109° 35´ length, 850 meters over sea level) In each farm, parturition were grouped, thus, most of the goats had the comparable milking days; the lactation stages considered were from 30 to 60 days postpartum and 90 to 110 days postpartum. Social questionary was performed to determine income and cheese facilities.
Feeds and milk sampling and analysis
In May and August of each year, milk samples (100mL) were collected once a day for three consecutive days, in a sterilized plastic falcon tube, refrigerated at 3°C and transported to the laboratory. An aliquot of each sample was analyzed for fat, protein, and lactose (Milko Scan™ 133B, Foss Matic, Hilleroed, Denmark) while another one was refrigerated at 3 °C for 4h, frozen at -21 °C for 48h and then lyophilized. Contemporary, in EG and SG system pasture samples were collected as follows: grass of four different areas (2.5m2 each) was cut at 3cm from the ground; once weighed, 4 representative samples (1kg each, obtained balancing the amount from the 4 different areas), legume leaves were cut after goat grazing, both sample were air-oven dried at 65 °C, milled through a 1mm screen and analyzed according to AOAC [16] for dry matter (DM, ID 934.01), crude protein (CP, ID 984.13), ether extract (EE, ID 920.29); the structural carbohydrates were also determined [17] and nutritive value (UFL=1700 kcal of net energy for lactation) was calculated [18].
Among the 84 farms included in the trial, 25.3% were in FC (359 animals) fed corn silage, alfalfa hay, and commercial balanced concentrate (16% CP; 500 to 1,000g/ head/day); in both extensive (EG) and supplemented (SG) grazing was a mixture of grasses; Bouteloua curtipendula, Chloris virgata, Bothriochloa saccharoides, Leptochloa dubia, Rhyncheltythurum roseum, Panicum obtusum, Bouteluoa repens, Aristida adscensionis, Setaria parviflora, Urochloa fasciulata, Pennisetum ciliare, legumes: Prosopis leavigata, Acacia farnesiana, Acacia schaffneri, Mimosa biuncifera, Shrubs: Celtis pallida, Psilactis brevilingulata, Jatropha dioica, Zalazania augusta var. augusta, Verbasina serrata and Cactus: Opuntia affasiacantha, O. amyctaea, O. cretochaeta, O. hytiacantha, O. robusta, O. streptacanta, O. tomentosa and O. imbricata Group EG (30%, 426 does) was permanently on rangeland while SG (44.6%, 632 heads) was partially on rangeland supplemented with a commercial balanced feed (18% crude protein; 500 to 1,000g/head/day). Average daily milk yield was significantly (P<0.05) different among groups: 1.6±.212kg (FC), ,950 ±.272kg (SG) and .720±.153kg (EG).
Milk fatty acid analyses
Total fat of milk lyophilized samples was separated by a mixture of hexane isopropane (3/2, v/v), according to Hara and Radin [19]. Transmethylation of fatty acids was performed by the base-catalysed procedure described by Christie [20] and modified by Chouinard [21]. FAME were quantified by gas chromatography (GC) using a CP3380 chromatograph equipped with a split injector, flame ionization detector (FID) and auto sampler CP 8400. A DB23 column (30mx 0.25mm i.d.) with a film thickness of 0.25μm was employed. Nitrogen was used as carrier gas at a flow rate of 30ml/ min. Temperatures column was held for 1min at 120 °C, then programmed at rate of 10 °C/min to 200 °C and held for 5 °C/min to final temperature of 230 °C, temperature injector and FID were 250 °C and 300 °C, respectively. Integration for each fatty acid was performed by a Varian Star Chromatography Workstation Software. Identification of the peaks was made on the basic of the retention times of standard methyl esters of individual fatty acid (FAME mix C4-C24#18919-1 AMP). The final concentration of FAME was expressed as mg/100g of milk.
Oxidation
HPLC analysis
A high-performance liquid chromatography (HPLC, Agilen) with UV detector and autosampler, equipped with a binary pump, and a C8 RP column (ZORBAX Eclipse, XDB C8, 4.6x150mm 5μm) was used. A volume of 20μL of sample was injected, the solvent flow rate and the oven temperature were 2mL/min and 50 °C, respectively, and a wavelength of 210nm. A calibration curve was prepared using cholesterol (Sigma-Aldrich) from 0 to 1000μg as standard, resuspended in the mobile phase and a running time of 5min. For α-tocopherol determination present in the samples, the methodology described above was applied, in this case the wavelength be 292nm and an oven temperature of 35 °C. A calibration curve of 0 to 1000μg of α-tocopherol was use, which was be resuspended in the mobile phase and a running time of 6min. With cholesterol and alpha-tocopherol values, the equation proposed by Pizzoferrato et al. (22) was carried out.
Degree of antioxidant protection (DAP):
Where:
MA is the molar part of the antioxidant molecule lpha
tocopherol)
BO is the molar part of the oxidizing molecule (cholesterol) [22]
The experimental model was carried out under a completely randomized design, using a one-factor analysis of variance test and its Tukey tests with the Excel 2019 program.
DAP Calculation proposed as the tracing parameter, was calculated as the molar ratio between antioxidant compounds and a selected oxidation target:
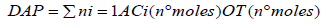
where AC is the antioxidant compound (α tocopherol), OT is the oxidation target (cholesterol) and i is the number of components.
To facilitate reading and comparison among samples, the DAP number is expressed in an exponential form (×10-3). This index can be used to evaluate the antioxidant protection of food products, selecting the proper antioxidants and oxidation target. For instance, α-tocopherol, β-carotene, and polyphenols represent a natural and particularly efficient antioxidant system in extra virgin olive oil; whereas linoleic acid, an oxidizable unsaturated fatty acid, can be selected as the oxidation target. Their combination in the DAP parameter is useful as a predictive index of oil sensitivity to oxidation during storage. In goat dairy products, only α-tocopherol was selected as the antioxidant because of the absence of detectable levels of β-carotene in goat’s milk, and cholesterol was the oxidation target because of the low content of oxidizable
unsaturated fatty acids in milk fat [22,23]. Moreover, even if less easily oxidizable than unsaturated fatty acids, cholesterol is a molecule usually charged, especially in the oxidized form; cholesterol oxides are responsible for heart disease in humans [24]. The effectiveness of the DAP parameter to distinguish milk and cheese from grazing and zero-grazing animals was evaluated in experiments 1, 2 and 3; DAP’s relationship with actual herbage intake was evaluated in experiment 4
Degree of antioxidant protection (DAP):
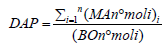
Where:
MA is the molar part of the antioxidant molecule (alpha
tocopherol)BO is the molar part of the oxidizing molecule
(cholesterol) [22]. For instance, α-tocopherol, β-carotene,
and polyphenols represent a natural and particularly efficient
antioxidant system in extra virgin olive oil; whereas linoleic
acid, an oxidizable unsaturated fatty acid, can be selected as the
oxidation target. Their combination in the DAP parameter is useful
as a predictive index of oil sensitivity to oxidation during storage
[24,25]. In goat dairy products, only α-tocopherol was selected
as the antioxidant because of the absence of detectable levels of
β-carotene in goat’s milk, and cholesterol was the oxidation target
because of the low content of oxidizable unsaturated fatty acids in
milk fat [22,23].
Moreover, even if less easily oxidizable than unsaturated fatty acids, cholesterol is a molecule usually charged, especially in the oxidized form; cholesterol oxides are responsible for heart disease in humans (The effectiveness of the DAP parameter to distinguish milk and cheese from grazing and zero-grazing animals was evaluated in experiments 1, 2, and 3; DAP’s relationship with actual herbage intake was evaluated in experiment 4. Finally, a social study was performed dividing farmers by income in three sectors, high, medium, and lower income. Cheese factories were classified according to the volume of manufactured milk, curdling system and maturation with or without the presence of a cellar.
Statistical Analysis
All the data were analyzed using a one-way ANOVA design. Data analysis was carried out using the General Linear Model Procedures (Stat graphics-Centurion), calculated with Statistical Analysis System [26].
Results
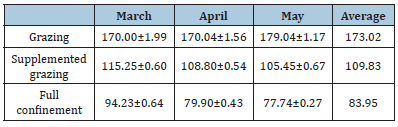
Results of this study show that the cholesterol levels, alphatocopherol, and the degree of antioxidant protection were mostly constant among the farms sampled inside the same system (Table 1-3). Concentration of alpha-tocopherol in milk increased from 170 to 179.04μg/100g in the month of May for grazing animals (P), decreasing from 115.25 to 105.45μg/100g and from 94.23 to 77.74μg/100g for PS and E treatments respectively (Table 4).
Table 1:Levels of cholesterol, alpha-tocopherol, and degree of antioxidant protection (DAP) in milk from goats from different feeding systems EG. SG. FC g systems.
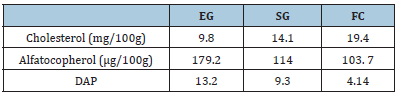
Table 2:Two independent samples t-test----(n=111) for differences in exchange of medical gloves for dental hygiene students by gender.Levels of cholesterol, alpha-tocopherol, and degree of antioxidant protection (DAP) in milk from goats from different management (EG; SG;FC) under supplemented grazing systems (SG).
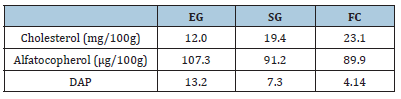
Table 3:Average levels of cholesterol in goat’s milk in March, April, and May (mg/100g).
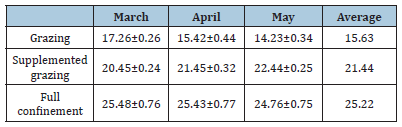
Table 4:Average levels of alpha-tocopherol in goat’ milk in March, April, and May (μg/100g).
Similarly, the cholesterol level in milk during the dry period was higher (p<0.05) in those animals fed under stable systems with diets rich in starches (25.22mg/100g), compared to with those who consumed a greater quantity of forages (Grazing (15.63mg/100g), Grazing + Supplement (21.44mg/100g) (Table 5). In the present study, the degree of antioxidant protection (DAP) had a statistically significant increase between treatments (Table 6), with the highest value being observed in Grazing (13.2), followed by the SG (7.3) and full confinement (4.4) groups.
Table 5:Diet chemical composition (% DM) and nutritive value (MUF/kg DM) in exclusive grazing (EG), supplemented grazing (SG) and confinement (FC) system.
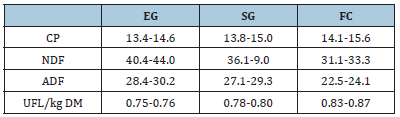
CP: crude protein; NDF: neutral detergent fiber; ADF: acid detergent fiber; UFL: net energy for lactation.
A. Milk yield: Average milk yield was kg 1.6±.212 vs 0.950±.272 vs 0.720±212kg, for EG, SG and FC respectively Milk chemical composition was unaffected by treatment (Table 6).
Table 6:Milk chemical composition (g/kg).
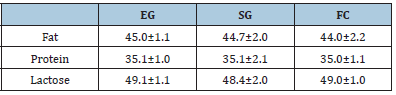
EG: Exclusive Grazing; SG: Supplemented Grazing; FC: Full Confinement.
B. Milk fatty acid profile: Myristic (C14:0), margaric (C17:0) and stearic (C18:0) acids, as well as total SFA, were significantly (P<0.05) higher in milk from FC than SG and EG. Group EG showed the lowest value of palmitic acid (C16:0) (28.12g/100g) statistically different (P<0.05) from both SG (30.00g/100g) and FC (32.13g/100g). The other SFAs were not significantly different among the breeding systems (Table 7).
Table 7:Milk saturated fatty acids (SFA) profile (g/100 g fat)
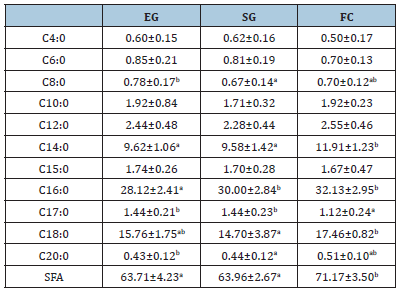
EG: Exclusive Grazing; SG: Supplemented Grazing; FC: Full Confinement.
Means with different letters indicate differences (P<0.05) among breeding systems. Milk from SG showed the highest concentration, even if not significantly different, of unsaturated fatty acids (UFA): 35.76g/100g vs 35.09g/100g vs 34.62g/100g, for SG, EG and FC, respectively. Concerning the monounsaturated fatty acids (MUFA), milk from EG had the highest concentration (32.35g/100g) compared to FC (31.42g/100g) and SG (32.27g/100g), but, again, the differences were not significant. Similar results were found for the polyunsaturated fatty acids (PUFA) concentration with no statistical difference among the breeding systems. The most representative acid in milk from all the systems was the oleic acid (C18:1) followed by linoleic acid (C18:2) and palmitoleic acid (C16:1) (Table 7).
Table 8:Milk unsaturated fatty acids (UFA) profile (g/100 g).

EG: Exclusive Grazing; SG: Supplemented Grazing; FC:
Full Confinement;
MUFA: monounsaturated fatty acids; PUFA:
polyunsaturated fatty acids;
UFA: unsaturated fatty acids; ω6/ω3: omega6/omega3
ratio.
The ω6/ω3 ratio was significantly (P<0.05) different among the systems: group FC had the highest value (6.21:1) followed by SG (3.35:1) and EG (2.07:1). This result was mainly due to the different linolenic acid (C18:3) concentration among the groups. Concerning ω6 FA, a significant lower concentration (P<0.05) was found for linoleic acid (C18:2) in milk from EG compared to those from the other systems (Table 8).
Means with different letters indicate differences (p<0.05) among management systems. Farmers income did not affect milk quality, even that different maturation and cost of cheese was significant different, source of feeding was the main source of cheese quality. Farmers income is a main source of cheese quality due to better cheese factories, however feeding systems was the main variable for cheese quality even considering taste.
Discussion
Herbage intake was estimated based on herbage mass, measured on 2×2m of pasture before and after grazing [19-22]. The real intake of grazed herbage for each group was 0 (control fed on commercial concentrate), 450, 600, and 1,000g of DM/d, and the contribution of grazing to the diet in each treatment was calculated as a percentage of the maximum actual herbage intake [from 0g/d (0% grazing) to 1,100g/d (100% grazing). Linear equation (y=a + bx) fits the experimental points with a high correlation coefficient (R2 = 0.97) and can be utilized as an analytical calibration curve. Replacing the variable y with the DAP threshold value, able to differentiate “pasture” products from “stabled” ones (DAP = 7.0- 3), the x value can be calculated [17,18]. This value (15%) can be considered the limit of DAP detection if the same amounts of freshly cut pasture herbage were ingested indoors, and not grazed by animals, the linear fitting of DAP values vs. the amount of cut herbage intake would show a lower correlation value (R2 =cheese commercial quality), contributing to price setting and probably to animal well-being. Moreover, the DAP parameter was found to be related to oxidative reactions, the main determinant of quality loss in food: the higher the DAP value, the greater the product stability and safety, considering the risk related to the intake of cholesteroloxides in humans as discussed by Pizzoferrato et al. [22].
Michoacan , grazing showed the highest DAP when compared with two management systems as full confinement and supplemented grazing (31); results are in agreement with previous data obtained in another study carried out in goat cheese by Pizzoferrato et al. [22], in Italy, where they reported data with the same trends, having in their work four treatments in which the GPA of goat’s milk and cheese was evaluated, obtaining higher values in the treatments where neither grains nor commercial concentrates were offered: DAP were 11.3 on full grazing, 9.8 supplemented grazing, supplemented grazing with concentrate, 10 goats in each observation were feed full commercial concentrates in confinement [27].
Previously Pizzoferrato et al., [22] reported that no significant differences were found in their samples in cholesterol levels in goat milk, with cholesterol values of 14.7 for grazing, 11.8 for supplemented grazing, 12.8 for grazing with commercial supplement and 12.8 in full confinement. In the present study, the cholesterol levels in goat’s milk were: 15.63 in grazing, 21.44 in grazing + supplement and 25.22 in confinement, showing a slight increase in cholesterol between the different treatments being higher in full confinement, this difference could be due to the milk source since those researchers carried out his study on goat’s milk.
The data obtained from alpha-tocopherol in this work demonstrated significant differences in the treatments where there is supplementation, having an average of 173.03 in grazing, 109.83 in supplemented grazing and 83.96 in full confinement. Similar results to those reported [22] in goat milk where the highest value of alpha-tocopherol is 154.9 in grazing, 118.9 in supplemented grazing, 125.2 in grazing with concentrate and 77.1 in full confinement. In the study by Pizzoferrato et al. (22), samples were obtained for three years, and during these three years, the same trend was followed in the data for cholesterol, alpha tocopherol and FPG.
Similarly, in a study by Andrea Cabiddu et. al. [4] in Italy reported a higher amount of vitamin E, but also a higher amount of cholesterol in the milk of goats fed a diet high in forage. In this study, two feeding treatments were made: the first treatment determined a diet with low forage coverage, and I call it LH (low herbage cover), and the second treatment HH (high herbage cover) was a diet with high forage coverage and obtained results in HH of 3.66 and LH of 3.52 in vitamin E, showing that the feeding of goats with high concentrations of forage contained greater amounts of this vitamin.
In the case of cholesterol, higher amounts were obtained in the HH treatment, with values of 314, and in the LH treatment 294 were obtained. They also performed the GPA of their data, and a higher GPA was obtained in the second treatment 11.66 for HH and 11.97 for LH [23]. The foregoing can be explained by the presence of forage, in their case they used hay forage, a process that alters the antioxidant profile due to the plant’s desiccation, this being reported by Tornabmbe et al. (2010) where they obtained greater amount of alpha-tocopherol in diets with more than 40% green forage: This coincides with the similar parameters from this work since the goats were grazed with fresh forage, thus obtaining the best conditions to produce milk with high concentrations of alphatocopherol, and therefore have a higher GPA (Degree of Antioxidant Protection) as discussed before [28].
Another study conducted by Claps et al. [10] studied the degree of antioxidant protection of organic milk, reporting that their indoor feeding data showed the lowest values compared to grass-fed groups, and the groups fed with grass + corn grain in the concentration of vitamin E, A and β-carotene. The Degree of Antioxidant Protection showed an increasing trend in organic milk. Grazing + maize feeding was associated with the highest level of FPG (9% higher than grass-only feeding and 79% higher than indoor feeding) demonstrating a very similar trend to that found by Cabbidu et al. [4] in his study in Italy, and that we attribute to feeding the animals with hay forage; however, the prevalence follows in all reported cases where diets based on fresh forage denote a higher GPA.
Indeed, Park et al. [28], Jensen [29], Chapkin [30] and Banskalieva et al. [31] discussed that fat and cholesterol have worldwide increased in human’s diet, thus becoming a serious health risk due to coronary and vascular problems. According to these authors, the consumption of saturated fatty acids, particularly lauric (C12:0) myristic (C14:0) and palmitic (16:0), are related to hypercholesterolemia due to an increase in plasma low density lipoproteins (LDL) while stearic (C18:0) and oleic acid (C18:1) decrease LDL and increase high density lipoproteins (HDL), favoring liver formation of very low density lipoproteins (VLDL) that allows cholesterol to be transformed to gall bladder salts (25). Data in the present trial confirmed this low potential hypercholesterolemic effect of milk from grazing animals; in fact, milk from exclusive grazing had significantly lower contents of both myristic (C14:0) and palmitic (16:0) acids, and milk from supplemented grazing only of palmitic acid than that from full confinement system [26].
Despite a similar content of oleic acid (C18:1), milk from EG and SG showed 50% higher content of linolenic acid (C18:3) than that from FC group. Some studies reported modifications of milk fatty acid profile as a function of animal’s diet: in particular, a decrease of C16:0 and an increase of C18:0 and C18:1 content was observed in cows grazing pasture compared to cows fed a total mixed ration [27,28].
Concerning ω6 and ω3 PUFA, it has been shown that milk contents of both linoleic acid (C18:2, ω6) and linolenic acid (C18:3, ω3) are affected by the feeding system [27]. Similarly, in the present study, the highest content of linoleic acid was in milk from FC while that of linolenic acid in milk from EG. Decreasing milk ω6/ω3 ratio showed several beneficial effects for human health [5,15], particularly with values lower than 4, since higher levels could modify the beneficial effects of ω3 [14,15]. In present trial, the relationship between breeding system and milk ω6/ ω3 ratio was shown feeding animals with higher quantity of concentrates increased milk yield and ω6/ω3 ratio. Similar results were reported by Salado et al. [32], in a study aimed to evaluate the effects of diets with different levels of concentrate (3.5,7.0 and 10.5kg/day) on milk yield and quality of grazing dairy cows in early lactation [30,31,33]. These authors registered higher milk yield and protein in groups fed 7.0 and 10.5kg/day than in group fed 3.5kg of concentrate. In contrast, milk fat did not differ among the groups and, even if the potential hypercholesterolemic fatty acids of milk (C12:0 to C16:0) did not change by increasing concentrate intake, linolenic acid decreased and the ω6/ω3 ratio increased in groups fed higher amounts of concentrate. Corazzin et al. [25] evaluated the effect of concentrate supplementation (High: 3.0kg/head/d vs. Low: 1.5kg/head/d) on milk fatty acid profile of Italian Simmental dairy cows grazing on alpine pasture. Low milk showed higher concentration of linolenic acid and total PUFA than High milk [34].
Recently, Santa-Ana et al. [35] and Galina et al. [36] compared two breeding systems for goats, full confinement or grazing: milk from animals fed on pasture showed higher PUFAs and MUFAs and lower SFAs with a significant reduction of atherogenic index, thus presumably more beneficial for human health. Musco et al. [27] evaluated the effects of a feeding strategy (based on the use of outdoor paddocks; forage: concentrate ratio at least 70:30; forage including at least five different herbs; and no silages) in dairy cows on milk yield and chemical composition, and blood metabolic profile, including the evaluation of oxidative stress. These authors reported that the proposed feeding system was able to increase milk quality, mainly in terms of fats quality, without negative effects of animal health. Animals fed higher forage: concentrate diet were able to maintain body homeostasis by changing metabolism despite the low energy diet and they showed a general improvement of oxidative status, probably due to an improvement of the biological antioxidant potential. All these results showed that, even considering the differences among ruminants, management on grazing is the key component to improve omega relationship.
It is generally accepted that the quality of the cheese is the product of the processing, type of curd, management of the curd, and maturation system, however in the present study it was shown that the feeding system is the main variable that determines the quality of the cheese, similar results to those observed recently by Rubino and Galina [36].
Conclusion
Feeding system resulted as a fundamental aspect to determine the nutritional quality of milk, mainly related to its fatty acid profile [36-39]. Despite an increase of milk yield, the full confinement system showed a worsening of healthier parameters: ω6/ω3 ratio greater than 4: DAP was also significant higher in full grazing. Therefore, in response to the consumer demand for foods with high nutritional quality, the grazing systems must be encouraged and milk from animals with free access to pasture must be recommended. Finally, it is generally accepted that the taste quality of the cheese is the product of the processing, type of curd, and maturation system, however in the present study it was shown that the feeding system is the main variable that determines the taste of the cheese.
References
- Slots T, Butler G, Leifert C, Kristensen T, Skibsted LH, et al. (2009) Potential to differentiate milk composition by different feeding strategies. J Dairy Sci 92(5): 20157-2066.
- Tudisco R, Calabrò S, Cutrignelli MI, Moniello G, Grossi M, et al. (2012) Influence of organic systems on Stearoyl-CoA desaturase in goat milk. Small Rum Res 106: s37-s42.
- Tudisco R, Grossi M, Calabrò S, Cutrignelli MI, Musco N, et al. (2014) Influence of pasture on goat milk fatty acids and Stearoyl-CoA desaturase expression in milk somatic cells. Small Rum Res 122(1-3): 38-43.
- Cabbiddu A, Molie G, Decandin M (2017) Influence of the goat rearing system on the vitamin and pheolic composition of milk. (Influence of the goat breeding system on the vitaminic and phenolic composition of milk). Scienza e Tecnica Lattiero Casearia 68(3-6): 67-68.
- Galina MA, Pineda J, Higuera PR, Vázquez P, Haenlein G, et al. (2019) Effect of Grazying on the fatty acid composition of goat´s milk or cheese. Adv Dairy Res 7(3): 227.
- Galina MA, Piñón JO, Ortiz RA, Higuera PRI, Dela CH, et al. (2022) Effect of nutritional system on the degree of antioxidant protection of bovine Milk. Nov Res Sci 12(3): 1-7.
- Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 97: 55-74.
- Castel JM, Mena Y, Ruiz FA, Camuñez Ruiz J, Sánchez-Rodriguez M (2011) Changes occurring in dairy goat production systems in less favored areas of Spain. Small Rumin Res 96: 83-92.
- Galina MA, Osnaya F, Cuchillo HM, Haenlein GFW (2007) Cheese quality from milk of grazing or indoor fed Zebu cows and Alpine crossbred goats. Small Rumin Res 71: 264-272.
- Claps S, Galina MA, Rubino R, Pizzillo M, Morone G, et al. (2014) Effect of grazing into the omega 3 and aromatic profile of bovine cheese. J Nutr Ecology and Food Res 2: 1-6.
- Galina MA, Elías A, Vázquez P, Pineda J, López B (2016) Effect of the use of fermentation promoters with or without probiotics on the profile of fatty acids, amino acids and cholesterol of milk from grazing cows. Cuban J Agric Sci 50(1): 105-120.
- Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmaco 56(8): 365-379.
- Cavaliere G, Trinchese G, Musco N, Infascelli F, De Filippo C, et al. (2018) Milk from cows fed a diet with a high forage: Concentrate ratio improves inflammatory state, oxidative stress, and mitochondrial function in rats. J Dairy Sci 101(3): 1843-1851.
- Trinchese G, Cavaliere G, Penna E, De Filippo C, Cimmino F, et al. (2019) Milk from cow fed with high forage/concentrate ratio diet: Beneficial effect on rat skeletal muscle inflammatory state and oxidative stress through modulation of mitochondrial functions and AMPK activity. Frontiers in Phys 9: 1969.
- Colavilla G, Amadoro C, Mignona R (2014) Omega 6/omega 3 ratio and GPA in Noble Milk in Molise en R Rubino. The Noble Milk Model is another way possible. Anfosc Caseus, Italia. 118-128.
- Gomez CC, Bermejo LML, Loria Kohem V (2011) Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr Hosp 26(2): 323-329.
- (2012) AOAC Official Methods of Analysis. (19th edn), Association of Official Analytical Chemists: Arlington, VA, USA.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10): 3583-3598.
- Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90(1): 420-426.
- (1978) Alimentation Des Ruminants. INRA: Paris, France, p. 597.
- Christie WW (1992) A simple procedure of rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res 23(7): 1072-1075.
- Pizzoferrato L, Manzi P, Marconi S, Fedele V, Claps S, et al. (2007) Degree of antioxidant protection: a parameter to trace the origin and quality of goat's milk and cheese. J Dairy Sci 90(10): 4569-4574.
- Pizzoferrato L, Manzi P (1999) Effect of the antioxidant potential of goat cheeses: Preliminary results. Caseus 4: 28-33.
- (1996) SAS Institute Inc, Statistical Analysis System, User's Guide Statistics. (12th edn), Carly, North Carolina, USA, p. 320.
- Corazzin M, Romanzin A, Sepulcri A, Pinosa M, Piasentier E, et al. (2019) Fatty acid profiles of cow’s milk and cheese as affected by mountain pasture type and concentrate supplementation. Animals 68: 1-13.
- Delaby L, Peyraud J, Delagarde R (2001) Effect of the level of concentrate supplementation, herbage allowance and milk yield at turn-out on the performance of dairy cows in mid lactation at grazing. Animal Sci 73(1): 171-181.
- Musco N, Tudisco R, Grossi M, Mastellone V, Morittu VM, et al. (2020) Effect of a high forage: concentrate ratio on milk yield, blood parameters and oxidative status in lactating cows. Anim Prod Sci 60(12): 1531-1538.
- Park WY, Juárez M, Ramos M, Haenlein GFW (2016) Physico-chemical characteristics of goat and sheep milk. Small Rumin Res 68(1-2): 88-113.
- Jensen RG (2000) Fatty acids in milk and dairy products. In: Chow C (Ed.), Fatty acid in foods and their health implications USA. Marcel Dekker, US, pp. 109-124.
- Chapkin RS (2000) Reappraisal of the essential fatty acids. In: Chow C (Ed.), Fatty acid in foods and their health implications USA. Marcel Dekker, US, pp. 557-568.
- Banskalieva VV, Sahlu T, Goetsch AL (2003) Fatty acid composition of goat muscles and fat deposits: A review. Small Rumin Res 37(3): 255-268.
- Salado EE, Bretschneider G, Cuatrin A, Descalzo AM, Gagliostro GA (2017) Milk yield and composition and pasture ruminal digestion in grazing dairy cows receiving three levels of energy concentrate supplementation. Agricultural Sci 8(10): 1135-1156.
- Zlatanos S, Laskaridis K, Feist C, Sagredos A (2002) CLA content and fatty acid composition of Greek Feta and hard cheeses. Food Chem., 78(4): 471-477.
- Galina MA, Ortíz-Rubio MA, Guerrero CM, Vazquez P, Pineda LJ (2016) Effect of feeding management on the nutritional composition of artisan soft cheese made with ewe´s J Nutr Ecology Food Res. 3(1): 36-42.
- Santa-Ana A, Bessa R, Alves S, Medeiros AN, Costa RG, De Sousa Y, et al. (2019) Fatty acid, volatile and sensory profiles of milk and cheese from goats raised on native samiarid pasture or in confinement. International Dairy Journal 91: 147-154.
- Rubino R, Galina M (2020) A new method to take advantage of the diversity of Mexican cheeses. Open Door Editors, ISBN 978-607-8640-82-9 p. 56.
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE (1999) Conjugated linoleic acids alter milk fatty acid compo-sition and inhibit milk fat secretion in dairy cows. J Nutr 129(8) :1579-1584.
- Kelly ML, Kolver ES, Bauman DE, Van Amburgh ME, Muller LD (1998) Effect of intake of pasture on concentrations of conjugated linoleic acid in milk of lactating cows. Journal of Dairy Science 81(6): 1630-1636.
- Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. (1991) Lancet 338(8773): 982-92.
© 2023 Galina MA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






