- Submissions

Full Text
Novel Research in Sciences
Technical Analysis for the Detection of BPA in Food Plastic Packaging
Li Yinxuan*
State Key Laboratory of Dairy Biotechnology, Shanghai Engineering Research Centre of Dairy Biotechnology, Bright Dairy & Food Co., Ltd, Shanghai, 200436, China
*Corresponding author: Li Yinxuan, Bright Dairy Co. Ltd, State Key Laboratory of Dairy Biotechnology, Shanghai Engineering Research Centre of Dairy Biotechnology, Bright Dairy & Food Co., Ltd, Shanghai, 200436, China
Submission: October 11, 2022;Published: October 19, 2022
.jpg)
Volume12 Issue2October , 2022
Abstract
Bisphenol A is widely used in the processing of plastic products and as an additive in the production of plastic packaging it can be transferred from the packaging to food and thus become a health hazard. It is therefore important to strengthen the inspection of BPA and to try to construct a more rapid and accurate testing technique with low detection limits in the process of product testing. Currently, the main techniques for the detection of BPA are polarimetric analysis, molecular imprinting, electrochemical analysis, fluorescence spectrometry, enzyme-linked immunosorbent technique, chromatography and chromatography-mass spectrometry. This paper summaries the above techniques and the current status of research on BPA detection and describes the expectations for the future development of detection techniques.
Keywords: Food packaging; Materials; Inspection and detection of bisphenol A; Analysis
Introduction
Food safety is one of the most important public health issues in society today and is expressed in the effects of toxic and harmful substances in food on human health. Food packaging is an essential part of the marketing and distribution of food, and therefore the safety of packaging materials is inextricably linked to food safety
Bisphenol A (BPA), which consists of two phenol groups in the opposite position by replacing the two hydrogen atoms above the middle carbon atom of propane, is a very important chemical raw material in chemical production and is widely used in plastic products that can be accessed on a daily basis, such as food packaging, beverage packaging, milk bottles and water bottles. Since BPA is an exogenous endocrine disruptor, as an additive in the preparation of plastic packaging products, it can migrate into food through plastic food packaging and accumulate in the human body, thus posing a health hazard [1]. BPA can cause obesity [2], diabetes, male infertility [3], developmental disorders [4], and kidney disease [1]. Many countries have enacted laws to ban BPA in baby food packaging materials [5], and the use limits for BPA in everyday products are constantly being reduced [6,7].
This shows that it is vital to improve the testing of BPA and to improve the safety of food packaging materials. At the same time, it is worthwhile to further study how to establish more rapid and accurate detection methods with low detection limits in the testing of food packaging materials. This paper summaries the current BPA detection methods and describes the current state of research.
Pre-Treatment Prior to Testing
Sampling of food packaging materials
Firstly, a scientifically sound food simulating solvent should be selected. This is to simulate the migration of BPA during contact between common food products and packaging materials. Depending on the type of food, the food simulating solvents are classified differently and a generally a mixture of several different solvents. In the immersion test of food simulating solvents, China has developed the “General Rules for the immersion test of packaging materials and their products for food”, which clearly stipulates that various products and samples need to be soaked in different solvents such as water, 20% ethanol and 4% acetic acid for 30min at 20 °C. After the food simulating solvents are mixed, they are heated and concentrated to fix the volume before the testing work is carried out. Among them, it needs to be noted that after the migration of BPA in food packaging materials, the corresponding sealing treatment should be done to avoid the volatilisation of the detected substances.
Next, the leaching of the food packaging material should be carried out. The cleaned and cut samples are first soaked in pure water for about 15min, then dichloromethane is added and stirred with a glass rod for about 2min until the dichloromethane is dissolved and 15ml of methanol liquid is added to help the precipitation of the polymeric compound. After standing for 20min, a centrifugation operation is carried out and the supernatant after centrifugation is selected, blown dry with nitrogen and then filtered by adding methanol to dissolve again to obtain the sample to be analysed. Thirdly, the extraction process is carried out using accelerated solvents. The packaging material was cleaned and dried, then ground in a freezer mill for 30min and extracted using accelerated solvents. Dichloromethane was used as the extractant and the resulting extract was then concentrated by a rotary evaporator.
Finally, the extraction method for Bisphenol A was chosen. Due to the strong molecular polarity of BPA, stronger solvents can be used to extract BPA from packaging materials. extraction methods include soxhlet extraction, ultrasonic oscillation extraction, water bath oscillation extraction and microwave assisted acetone extraction. The first three methods all require a certain amount of time to make the extraction solution concentrated in methanol and several iterations of extraction are performed..
Extraction methods for Bisphenol A
Soxhlet extraction: Soxhlet extraction as the main way of solid sample pretreatment, is considered the most versatile way of extraction of organic pollutants, but in the actual operation, there is a longer operating time, the need to use the machine extractant more and other deficiencies. In practice, Soxhlet extraction of BPA is more common, with extractants such as methanol, methylene chloride and other solvent mixtures.
Ultrasonic extraction: Ultrasonic extraction is based on the principle of using ultrasonic air to bring the solid sample into rapid and complete contact with the extraction solvent, so that the organic material can be dissolved in the solvent quickly and thus shorten the extraction time. The advantages of ultrasonic extraction are that the soaking time is reduced and no heating is required to maximise the properties of the sample, but the sample recovery is significantly lower than that of Soxhlet extraction.
Accelerated solvent extraction: Accelerated Solvent Extraction (ASE) involves subjecting the extract to a certain temperature and pressure to accelerate the dissolution of the substance and the diffusion of the solvent through the use of high temperature and pressure to improve extraction efficiency.
Microwave-assisted acetone extraction:Microwave-assisted acetone extraction can be used to extract BPA from paper-based packaging. The sample is placed in the inner tank of the microwave extraction tank, acetone is added and the extraction is carried out according to the selected microwave extraction conditions. After the extraction is completed, the extraction residue is washed with acetone, and after the extraction solution is cooled to room temperature, the extraction solution is then mixed with the washing solution and concentrated by rotary evaporation [8]. The extract solution to obtain the methanolic solution is concentrated and several iterations of the extraction are performed.
Testing Methods
As the society pays more attention to food safety, research on BPA detection methods is being enriched. Current methods for the analysis and testing of BPA include polarimetry [9], sensor methods [10], molecular blotting, electrochemical analysis [11], fluorescence spectrometry, enzyme-linked immunosorbent assay [12], Gas Chromatography (GC), High Performance Liquid Chromatography (HPLC), and chromatography-mass spectrometry coupling, including Gas-Mass Spectrometry (GC-MS) and Liquid- Mass Spectrometry (LC-MS).
Polarimetric methods
Polar spectrometry is mainly carried out by using the principle that the reaction of bisphenol A with nitric acid produces nitro compounds that appear as distinct second order derivative adsorption waves on the oscillometric polar spectrum, and the peak current is linearly related to the content of bisphenol A within a limited range. Zhang WD et al. [13] established a method for the determination of bisphenol A by single sweep oscillometric polarography. the concentration of NaNO2 was selected as 0.7mol/L, the temperature was 95 °C, the reaction time was 60min, the linear equation was determined, the limit of detection was 2ng/ ml, and the analytical recoveries were 80.2%~118.7% with RSDs of 2.2%~5.2%. The method is fast, simple and accurate, and its results are not significantly different from those of the HPLC method, which is suitable for the determination of BPA residues in food packaging materials.
Sensor method
A sensor is an analytical tool that uses an immobilized material as a recognition element combined with an appropriate physicochemical transducer and signal amplification device, Tsuru N et al. [14] used a quartz crystal microbalance for the determination of BPA with a detection limit of 100ng/ml, which can also be combined with ionophore resonance to improve the proactivity of BPA detection in food packaging materials. The current design in BPA detection introduces the cross-linking method of chitosan and graphene sheets as a sensor for BPA, with a detection range of 10-1300nmol/L, ensuring the accuracy of BPA detection [15].
Molecular blotting method
Molecular imprinting technique is to use the target molecule to be separated as a template, combine the functional monomer with the template molecule after adding crosslinker and initiator to form a polymer, and then use certain physical and chemical methods to make the template molecule detach to prepare a polymer material with specific memory ability for the target molecule. Zhang J et al. [16] prepared molecularly imprinted polymers selective for bisphenol A by electropolymerizing using bisphenol A as the template molecule and o-amino thiophenol as the monomer. The response characteristics of the sensor to BPA were investigated by cyclic voltammetry, and the results were linear with a detection limit of 2×10-7mol/L.
Electrochemical analysis
Electrochemical analysis is one of the important components in the detection of BPA in food packaging materials. It is a method that combines qualitative and quantitative instrumental testing and analysis based on the electrochemical properties of substances in solution and their change patterns by measuring the relationship between conductance, electricity, current, potential, etc. Yuchun W et al. [17] used functionalized single-walled carbon nanotubes to modify the catalytic active centre to have a large effective area in the electrode and to study the electrocatalysis of Bisphenol A on the electrode. It was found that after 100s of enrichment using a voltage of 0.2 V in a phosphate buffer solution at pH 6.5, the detection limit was 8.0nmol/L using cyclic voltammetry at a scan rate of 0.10v/s. Gao Y et al. [18] cross-linked β-cyclodextrin with single-walled carbon nanotubes to disperse it in the aqueous phase and formed a homogeneous and stable film adsorbed on the electrode, using The electrocatalytic effect enhanced its ability to oxidize BPA and reduced the oxidation electromotive force, and its detection limit could reach 1.0nmol/L.
Fluorescence spectrometry
Figure 1: Structure diagram of fluorescence spectrometer..
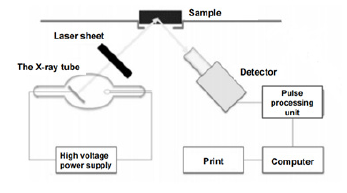
When using fluorescence spectroscopy to detect BPA in food packaging materials, the principle is to use the spectral properties of BPA itself for measurement, and to enhance its fluorescence intensity by adding an appropriate amount of β-CD without changing its properties. Yu-Yan Y et al. [19] used β-cyclodextrin to encapsulate BPA and quantify the BPA content in food packaging materials. At pH 4 and λex/λem=282/318nm for fluorescence spectrometry, the minimum detection limit was 0.12ng/ml, the linear range was 1-10ng/ml and the recovery reached 98%-102%, this method can provide food packaging materials with very low detection limits and high sensitivity (Figure 1).
Enzyme-linked immunosorbent assay
The ELISA technique has the advantage of high specificity and sensitivity in the detection of BPA. The competitive ELISA takes advantage of the fact that BPA cannot be used directly in immunized animals but can specifically bind to antibodies to detect BPA [20]. An indirect competitive enzyme-linked immunosorbent assay with a detection limit of 1ng/ml was established, and the recovery was 92.5%-107.7% with PC barrel water. Kongji W et al. [21] established a biotin-affinity amplified ELISA for the detection of bisphenol A. The sensitivity of the competitive ELISA was improved by combining the biotinylated secondary antibody with the primary antibody and adding the enzyme-labelled affinity, and the minimum detection limit was 0.05ng/ml. The method is simple in sample handling and highly sensitive.
Chromatography and chromatography-mass spectrometry
Figure 2: Chromatographic peaks of bisphenol A
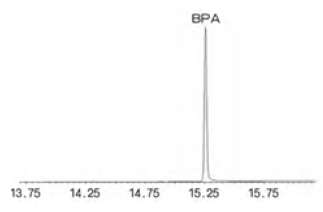
Chromatography and chromatography-mass spectrometry have improved the efficiency of the application of BPA detection methods. The chromatographic method has low detection limits, high sensitivity and good selectivity in structurally similar compounds. The main chromatographic methods currently used are gas chromatography, liquid chromatography and liquid mass spectrometry (Figure 2).
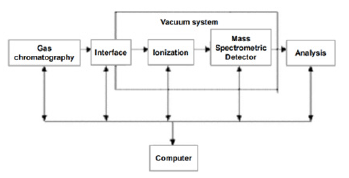
Gas Mass Spectrometry (GC-MS):HPLC-MS is currently the most widely used technique for the determination of Bisphenol A. Reversed-phase HPLC techniques can also be used, while many tests use HPLC-fluorescence for the quantification of Bisphenol A and gas-mass methods to detect the presence of the test substance. Since BPA can be trailed when the sample is directly detected by GC-MS and thus affect the sensitivity of the detection, derivatization of BPA is usually required in the specific operation, and the general derivatization operation methods include sialylation and acetylation. Huang B et al. [22] used iodomethane under alkaline conditions to carry out methylation derivatization of BPA specimens, which can reduce the polarity of BPA, and the phenolic impurities in BPA can be quantified and characterized by GC-MS, which is a fast and sensitive analytical method (Figure 3).
Figure 3:Schematic diagram of GC-MS.
Liquid Chromatography (HPLC):The use of liquid chromatography for the determination of bisphenol A can avoid the use of derivatizing reagents. Quan T et al. [23] chose the chromatographic conditions of a column-inertsil ODS-3, with a mobile phase of methanol-water (63:37, V:V), a flow rate of 1ml/ min, an injection volume of 10μl, a column temperature of 25 °C and an injector temperature of 15 °C. The maximum absorption wavelength was 228 nm. The maximum absorption wavelength was 228nm. Under the above conditions, the theoretical plate number of each group was not less than 3000, and the peaks of BPA were effectively separated from the peaks of other components.
Liquid Mass Spectrometry (LC-MS):Liquid Mass Spectrometry (LC-MS) is generally used for the determination of BPA in plastics such as polycarbonate (PC), polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and nylon (PA). Extraction was carried out by adding dichloromethane-methanol (90:10, v:v) to the samples and after extraction 20ml of methanol was added dropwise in a rotating state to re-precipitate the plastic. After filtering the sample, it was re-solubilised with methanol and determined by liquid chromatography. The minimum detection limit was 0.01mg/ kg with an average recovery of 90.1% to 102.5%. The HPLC and LC-MS detection methods do not require derivatization of BPA, but their resolution is not as strong as that of GC-MS, and the detection structure is easily interfered by the matrix, making the analytical results inaccurate.
Summary
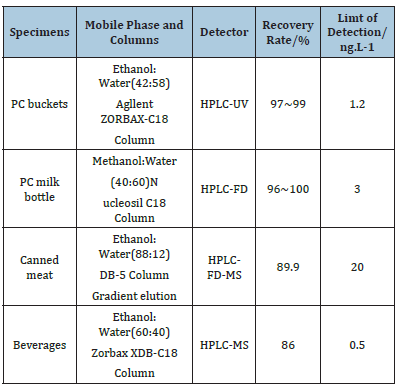
The polarographic method has the advantages of low detection limit, high detection efficiency and accurate detection, but it also needs to consider the influence of co-existing substances and the unstable nature of nitrosation products. The molecular imprinting method has good specificity of identification and stable reproducibility. Electrochemical methods have the advantage of being sensitive and rapid but have the disadvantage of not being able to accurately characterize samples for complex matrices. Fluorescence spectroscopy is low cost but is susceptible to interference and has low sensitivity. Enzyme-linked immunosorbent assays have the advantage of high specificity and sensitivity. Currently, the main methods for the determination of Bisphenol A in food packaging materials are chromatography and chromatography-mass spectrometry. These methods are characterised by low detection limits, high sensitivity and good selectivity. When using chromatography-mass spectrometry, it is possible to distinguish between compounds with similar structures and isomers (Table 1).
Table 1:HPLC method for detection of BPA in food and packaging materials.
Prospects
As China is a major food producing and consuming country in the world, the development and study of methods for the determination of BPA in food and food packaging materials is of great significance to strengthen the regulation and control of BPA in food and to improve food safety. In recent years, research on BPA has focused more on the dose of BPA in food packaging materials, but for the time being, studies on the hazards of low doses of BPA have been difficult to carry out, one of the major reasons being that low doses of BPA are present in large quantities in human life, and it is difficult to exclude the effect of this part of BPA on test results in tests. Despite this, many countries have taken steps to ban the production of baby bottles containing BPA, for example. In China, there is no clear national standard for the use of BPA, leaving the industry with no clear regulations to refer to. Therefore, the relevant departments and relevant enterprises in China should formulate scientific and reasonable laws and regulations and use standards as soon as possible to improve the current use of BPA in the market and life, to reduce the health and safety problems brought about by BPA. However, BPA plays an important role in food packaging and is widely used in human life, so increasing the research on alternative materials to BPA will be a direction for future development.
Acknowledgment
Sponsored by Shanghai Engineering Research Center of Dairy Biotechnology.
References
- Cabado AG, Aldea S, Porro C, Ojea G, Lago J, et al. (2008) Migration of BADGE (bisphenol A diglycidyl-ether) and BFDGE (bisphenol F diglycidyl-ether) in canned seafood. Food Chem Toxicol 46(5): 1674-1680.
- Corre LL, Moral LID, Besnard P, Chagnon MC (2012) Bisphenol A disrupts the intestinal lipid metabolism. Toxicol Lett 211(Suppl): S210.
- Meng XD, Yu JH, Yan YQ (2007) BPA-induced apoptosis in germ cells of male mouse and apoptotic molecular mechanism in vitro. J Toxicol 21(3): 201-204.
- Pinney SE, Mesaros CA, Snyder NW, Busch CM, Xiao R, et al. (2017) Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod Toxicol 67: 1-9.
- Mountfort KA, Kelly J, Jickells SM, Castle L (1997) Investigations into the potential degradation of polycarbonate baby bottles during sterilization with consequent release of bisphenol A . Food Addit Contam 14(6-7): 737-740.
- Chen D, Kannan K, Tan H, Zheng Z, Feng YL, et al. (2016) Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity. Environ Sci Technol 50(11): 5438-5453.
- Trasande L (2014) Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff (Millwood) 33(2): 316-23.
- Xiaoliang J, Bin W, Zhenghua X (2013) Determination of pentachlorophenol in paper packaging materials by microwave extraction and gas chromatography. China Paper 34(2): 22-24.
- Hongmei Y, Hao W, Yanqin L (2009) Determination of bisphenol A residues in beverages by liquid chromatography-tandem triple quadrupole mass spectrometry. Food Research and Development 30(12): 137-139.
- Quan T, Jing W, Yingli L(2013) Detection of bisphenol A in food and packaging materials. Chinese Journal of Food 13(3): 156-162.
- Jiang S-L, Huang S-B, Mai C-Huan, et al. (2012) Research progress of bisphenol A detection methods in food packaging materials . Packaging and Food Machinery 30(3): 55-59.
- Poorahong S, Thammakhet C, Thavarungkul P, Limbut W, Numnuam A, et al. (2012) Amperometric sensor for detection of bisphenol A using a pencil graphite electrode modified with polyaniline nanordrods and multiwalled carbon nanotubes. Microchim Acta 176(1-2): 91-99.
- Zhang WD, Ma ZD, Guo ZZ (2003) Polarographic determination of bisphenol A in food packaging materials. Analytical Chemistry 31(2): 249-250.
- Tsuru N, Kikuchi M, Kawaguchi H, Shiratori S (2006) A quartz crystal microbalance sensor coated with MIP for “bisphenol A” and its properties. Thin Solid Films 499(1-2): 380-385.
- Shankaran DR, Gobi KV, Miura N (2007) Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens Actuators B 121(1): 158-177.
- Zhang J, Xu L, Wang YQ (2009) An electrochemical sensor for bisphenol A based on molecularly imprinted electro polymeric membranes. Analytical Chemistry Research Bulletin 27(7): 1041-1044.
- Yuchun W, Zhaorong L, Qiaojuan G (2010) Detection of bisphenol A in food packaging materials by electrochemical analysis. Food Science 31(20): 303-306.
- Gao Y, Cao Y, Yang D, Luo X, Tang Y, et al. (2012) Sensitivity and selectivity determination of bisphenol A using SWCNT-CD conjugate modified glassy carbon electrode. Journal of Hazardous Materials 199-200: 111-118.
- Yu-Yan Y, Hui-Sheng Z, Mei S (2006) Determination of bisphenol A in food packaging materials by fluorometric method. Journal of Analysis and Testing 25(5): 99-101.
- Chunhong S (2008) Study on the determination of bisphenol A by enzyme-linked immunoassay, Jiangnan University, Wuxi, China.
- Kongji W, Meiping Z, Yuanzong L (2005) Determination of bisphenol A by biotin-affinity amplified enzyme-linked immunosorbent assay. Analytical Chemistry Research Bulletin 33(8): 1122-1124.
- Huang B, Xie MJ, Jiang Y (2002) Analysis of trace phenolic impurities in bisphenol A by gas chromatography. Petrochemicals 31(6): 476-478.
- Quan T, Yingli L, Jing W (2012) Determination of bisphenol A and its migration in PC milk bottles by high performance liquid chromatography. Food Science 33(22): 255-258.
© 2022 Li Yinxuan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






