- Submissions

Full Text
Novel Research in Sciences
The Impact of Sequencing Human Genome on Aging
Hameed Khan A*
National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) England
*Corresponding author: Hameed Khan A, National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), London University, England
Submission: September 22, 2022;Published: October 13, 2022
.jpg)
Volume12 Issue1October , 2022
Abstract
This abstract attempts to explain by comparing the sequence of the human genomes of senior citizen every year after age seventy, we can identify the emergence of new mutations responsible for causing old age diseases like Cancers, Cardiovascular Diseases and Alzheimer. If deleterious mutations are confirmed, early treatments will extend senior’s life beyond one hundred years. If we want to colonize Mars before this decade is over, we must extend human life so that human settlers using Mars as a base could launch unmanned spacecrafts in search of nearest exoplanets in the Milky Way Galaxy before this century is over. Although we age and die, Deoxyribose Nucleic Acid (DNA) neither age nor die; it keeps on passing information from generation to generation. Earliest unicellular life carries a single stranded DNA which grows by asexual reproduction by budding and making its own clones. In multicellular life form double stranded DNA in which homologous recombination takes place during sexual reproduction. The offspring of such union grows old and die. The laws of nature decide that all living creatures of double stranded DNA must die. If we want to defy Mother nature and extend human life span, we must seize the power from Mother nature and use the tools of genetic engineering to cut, paste, copy, and create a race of superior humans. If we want to protect, preserve, and spread human intelligence across the entire Universe, we must make every attempt to extend human life for the deep space travel. To colonize the exoplanets in the Milky Way Galaxy alone, we must travel at least half the speed of light while doubling the human life span.
A note to my readers
The Impact of Sequencing Human Genomes are a series of lectures to be delivered to the scholars of the National Youth League Forum (NYLF) and the International Science Conferences. NYLF scholars are the very best and brightest students selected from all over the USA and the world brought to Washington by Envision, an outstanding organization that provides future leaders of the world. I am reproducing here part of the lecture which was delivered at the International Science Conference that was PCS 6the Annual Global Cancer Conference held on November 15-16, 2019, in Athens, Greece
Special notes
I am describing below the use of highly toxic lethal chemical weapons (Nitrogen Mustard) which was used during WWI and developed more toxic weapons during WWII. I describe the use of Nitrogen Mustard as anti-cancer agents in a semi-autographical way to accept the responsibility of its use. When we publish research papers, we share the glory and use the pronoun “We” but only when we share the glory not the misery. In this article by adding the names of my coworkers, the animal handers, will share only misery. The Safety Committee is interested to know who generated the highly lethal Chemical waste, How much was it generated and how was it disposed. I accept the responsibility. The article below sounds semiautobiographical, it is, because I am alone responsible for making these compounds of Nitrogen Mustard, Aziridines and Carbamate. To get a five-gram sample for animal screening, I must start with 80 grams of initial chemicals for a four-step synthesis. To avoid generating too much toxic chemical waste, instead of using one experiment with 80 grams, I conducted 80 experiments with one gram sample, isolating one crystal of the final product at a time. The tiny amount of waste generated at each experiment was burned and buried at a safe place according to safety committee rules.
Keywords: Evolution; Biodiversity; Mutations; Genome sequencing; Drug design; AZQ
Introduction
It is the law of nature that all living creatures must die. A hundred years ago, there were people working on farms, factories, and offices, among millions only a handful are alive today and the rest die. Why people die and why they were all replaced by a new generation of people. In this article, I will attempt to explore if it is possible to slow down, stop and reverse aging process. If prayers answer, you should pray, here I will answer these questions on scientific and rational basis particularly on environmental and genetic basis. About a decade ago, the average human life span was about 70 years. Now, if you visit retirements homes, you find people living past age 70. Based on these observations, it appears that the average human life span should have been extended to 80 years. Because of good food, good medicine and good exercises, the human life span should be extended to 90 and beyond. By the end of the decade, our goal should be 100 years. Creating centenarian and super centenarian, should be our goal. Although the population of world has gone to about eight billions and we are adding 90 million additional people each year, we must continue to work on extending human life beyond one hundred years, because the seniors carry tremendous amount of knowledge and experience to share and train young people. As we plan to colonize Mars before this decade is over, we need to train an army of highly trained technicians on Earth and send them to Mars to grow new food, new fuel, and new medicine to help the next generation of the residents of Mars. Planet Earth should be reserved for very young and very old. Earth should be converted to a super luxury Disney World. It would be a training ground for the very young and a luxurious retirement home for the very old. During the next decade, an army of Robots will clear the Martian surface for human settlements. To work on Mars, humans between the ages of 18 and 58 will be trained and send to Mars to clean the surface for the next wave of human migrants arriving from Earth. Their training will prepare them to use Mars as a base to launch thousands of mini unmanned spacecrafts in search of exoplanets in the Milky Way Galaxy.
The Impact of Atmosphere on Aging
Early aging is caused by two factors: first is the external effect on our genes and the second is the internal effect on genes caused by the genetic make-up of the individuals inherited from parents. Among the external impacts includes such things as deprivations of food and water, extreme temperature or radiations, polluting chemicals in the environment, natural or manmade, which may disturb the body’s chemistry and infectious microorganisms which accelerating aging and death. The other major atmospheric impact on aging is the atmosphere of Earth itself. The atmosphere of Earth consists of 78 percent Nitrogen and 21 percent Oxygen. Oxygen is very reactive and toxic. Oxygen is so reactive that it reacts with Iron in the presence of water forming rust. The corresponding organ in humans is energy producing mitochondria. Hundreds of mitochondria are circulating in our blood providing Oxygen to ever cell in our body. More than 2000 mitochondria found in each liver cell alone. Mitochondria are usually located in the cytoplasm of cells where they generate energy using Adenosine Triphosphate (ATP) to empower cellular functions. Red Blood Cells are responsible for delivering oxygen to the body via hemoglobin. To supply Oxygen to hemoglobin, the ATP is broken to ADP (Adenosine Diphosphate) which is further broken to AMP (Adenosine Monophosphate). AMP is reconverted back to ATP by the enzyme phosphokinase in the presence of inorganic phosphate. To constantly provide Oxygen to every cell of our body, ATP is broken down to AMP which is converted back to ATP. It is a cyclic process and requires lots of Oxygen. Oxygen is extremely reactive, it damages the DNA, RNA, and protein. Residues of Oxygenated product are accumulated within the cell resulting aging.
The Impact of Genetic on Aging
Genetics require the transformation and segregation of traits over many succeeding generations. Using the techniques of genetic engineering, this article attempts to explores how should we conquer old age diseases and extend human life to produce centenarians and super centenarians in the age of Genetic Revolution. Let me provide some basic background. The entire book of life of all living creatures from a tiny blade of grass to mighty elephant including man, mouse, monkey, mosquitos, and microbes is written in four genetic letters called nucleotides. They are Adenine (A), Thiamine (T), Guanine (G) and Cytosine (C). A string of AT/GC base pairs is called the Deoxyribose Nucleic Acid (DNA). Out of four nucleotides, three nucleotides code for an amino acid called Codon. Different combination of four nucleotides gives sixty-four Codons. Sixty-one codons code for all 20 amino acids and the last three code serve as stop codons. Hundreds of codons join to form a gene. A gene is a unit of inheritance. Genes code for proteins. On a string of DNA, a gene has one start codon and three stop codons. The start codon of a gene is AUG (which codes for an amino acid called Methionine). There are three stop codons. They are UAG, UGG & UGA. Once the stop codon appears, DNA synthesis stops. In 1953, Morris Wilkins and Rosalind Franklin working in King’s College, London University, England, determined the crystallographic Structure of DNA by X-ray diffraction. Using their diffraction pattern data, Francis Crick and James Watson at the Cambridge University, England, determined the double helix structure of DNA which provides a copying mechanism of replication essential for Life to reproduce. It explained how the information is stored and copied in the double helix of DNA, a property only living creatures possess. The Age of DNA began with the discovery of double helix structure of DNA by Crick, Watson, and Wilkins for which they were honored with a Nobel Prize [1]..
The double helical DNA structure is transcribed into a single stranded of RNA (in RNA the less water-soluble methyl group in Thiamine T, is converted to more water-soluble Uracil U, by replacing Methyl group with a Hydroxyl group) which leaves the nucleus and moves into Cytoplasm where it is translated in Ribosomes into Amino Acids leading to proteins). When thousands to millions of AT/GC base pairs contain information to make a single protein, we call that portion of AT/GC base pairs a gene. The revolution of Genetic Engineering began in early seventy just over fifty years ago. Today, we are living in an age of genetic engineering, in an age of biotechnology, in an age of DNA. It is an age in which we can synthesize a gene, cut, paste, and copy a gene; we can take a gene apart and put it back together. With greatest ease, we can move a gene around at will from a man to mouse, from mouse to monkey and from monkey to microbes. The splicing of genes from one species to another has become so common, the technique is no longer considered revolutionary. Now, it is possible to synthesize a living creature from the raw material of DNA. With the evolutionary time given, species will have tried and discarded many different forms of allele or its component genes to secure its place within given environmental needs. The nucleus of each human cell carries 23 pairs of chromosomes. The first 22 pairs are called autosomal chromosomes. The last pair is not similar, and they are labeled as X and Y chromosomes. They are six linked chromosomes. Somatic mutations are quite limited. There are about six thousand mutations in our genome responsible for causing six thousand different diseases some of them are cystic fibrosis, sickle cell anemia, muscular dystrophy, Tay-sack disease caused by a defect in a single gene. Multiple defected genes such as diabetic, may be either dominant or recessive. Aging can be slowed down either by [1] replacing bad genes with good genes from the genome or by [2] designing novel drugs to shut off bad genes to treat old age diseases such as Cancers, Cardiovascular diseases, or Alzheimer.
Genetic Modifications to Slow Down Aging
Genes code for proteins. How genes specify the structures and functions of something as complex as the nervous system by receiving, processing, retrieving, and storing information with such as a fast speed, is beyond understanding currently. The earliest work on aging was done on a tiny worm called C. elegans because the language of life in all living creatures is written using the same four nucleotides from a tiny worm to mighty elephant including humans. The entire Cl elegans is made of a thousand cells. It reaches its maturity in three days. Average lifespan of C. elegans is thirteen days. It is grown in soil, and it is easy to maintain in the Lab by feeding on common bacteria such as E. coli. It is self-fertilizing worm that is it is hermaphrodite. It is made of six chromosome and carry 18,000 genes. Sequencing its genome identifies two genes which are CD-3 and CD-4 almost identical are responsible for causing program cell death occurred during embryonic development. If either gene is mutated, the worm fails to die and continue to live. During the study, it was also discovered that a gene called CV-9 reverse the action of CD-3 and CD-4. What is true for worm is also true for humans. As I said above since the language of life is based on the different combinations of the same four nucleotides that is A-T and G-C. We now discovered one of the secrets of aging in humans. During cell replication in humans, the chromosomes are shortened by a six-letter code (TTAGGG) called telomere at each replication. This telomeres loss is linked to aging. Now, an enzyme called Telomerase Reverse Transcriptase (TRT) has been discover which stops and reverse the loss of telomeres and could prevent aging. The gradual decay of the physiological processes which contributes to the progressive and extreme slowdown of the cell cycle is defined as Senescence. Senescence can irreversibly cause cessation of cell division of normally proliferating cells. Human cells become senescent from progressive shortening of telomeres due to cell division, stress, or oncogenes. Apoptosis is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to mutations causing characteristic cell changes and death. These changes include cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The key difference between apoptosis and senescence relies on the mechanism through which a living cell undergoes death and destruction. Apoptosis is a form of programmed cell death while senescence is the process by which cells irreversibly stop growth and division because of the ageing of cells..
As I said above, humans have 46 chromosomes. Each of our parents gives us 23 chromosomes via the egg or sperm, for a total of 46. Human genes are arranged along those 23 pair of chromosomes. The first 22 of these pairs, the chromosomes are identical. They are called autosomal. The twenty-third pair, called sex-linked, is not identical and is called X and Y chromosome that determines the individual’s gender. All those traits, which are controlled by genes on X and Y chromosomes, are called sex-linked traits. A fetus who inherits the two X chromosomes, as in XX, is a female. On the other hand, a fetus which inherits XY chromosome is male. If a gene residing on the X chromosome undergoes mutation, it does not exhibit the disease in female. The female becomes a carrier. This mutation does not have a problem because it has two X chromosomes. The unaffected chromosome protects the mutated chromosome. However, a male carrying the same defected chromosome is not protected and will exhibits underlying diseases. He will not have compensating additional chromosome to protect him from the disease. The mutation in effect becomes dominant. Mutated gene lying on the X and Y chromosome, leads to sex linked diseases like Hemophilia, Ducharme Muscular Dystrophy, Fragile X Syndrome. On the other hand, alteration in genes on any of the other 22 pairs of autosomal chromosomes may give rise to autosomal genetic diseases. Accumulations of mutations in autosomal chromosomes over years is responsible for causing old age diseases such as Cancer, Cardiovascular diseases, and Alzheimer. Recessive genetic diseases are almost always autosomal. Gene codes for protein. Whatever the origin of a defective gene, the disease arises from the abnormal proteins encoded by that mutated gene. The normal protein needed to carry out a critical function in the body is not available. If the protein is crucial during fetal development, its loss may give rise to what is called the developmental lethal disorder in which case it does not cause a disease per se. The fetus
is simply aborts, and the underlying cause is rarely pursued. If the fetus survives in the womb, it may be born and manifest symptoms of disease essentially from birth or diseases symptom may appear early part of the life.In modern times, many couple are well informed, and they come to see bedroom conceptions as dangerous and unnecessary risk. Homologous Recombination of 1,144 genes from mother and 231 genes from father, could produce unimaginable combination of genes which present great risk to the life of mother and her fetus. It would be easier to sequence the genome of egg and sperm before conception and use the healthy fertilized ovum for in vitro fertilization. If the couple has a family history of serious illnesses, their 8-celled embryo could provide a single healthy cell in the womb for the full-term fetal development. Each nucleus in our cell carries the entire blueprint of our whole body. But the result will be chaotic if every cell trying to create the whole person. Instead, our genetic DNA is regulated by a process called Epigenetic which determines which genes are expressed and the rest genes are shut off. A skin cell for example contains the blueprint of a liver cell as well and every other type of cell, but the Epigenetic marker tells the skin cell to produce skin cells only. All other genes are turned off. DNA methylation is an Epigenetic modification critical to normal genome regulation and development. Folic acid is known as Epigenetic agent. It methylates the DNA shutting off a gene. Methylation of the DNA sequence of a gene may turn a specific gene off, so that it does not make all other proteins. Gene activation can be restored by treating cells with 5-azacytidine, a potent demethylating agent. DNA methylation typically acts to repress gene transcription. In short, methylation turns genes “off” and demethylation turns genes “on.”
The total genetic information that makes our book of life is called Human Genome. The reading of Human Genome.is considered essential if we were to identify mutations and to design novel drugs to treat all six thousand diseases in our Genome[2-5]. The sequencing of the Human Genome will answer some of the most fundamental questions we have asked ourselves since the dawn of human civilization. What does it mean to be human? What is the nature of our memory and our consciousness? Our development from a single cell to a complete human being? The biochemical nature of our senses; the process of our aging. The scientific basis of our similarity and dissimilarity, Similarity that all living creatures from a tiny blade of grass to the mighty elephant including man, mouse, monkey, mosquitos, and microbes are all made of the same chemical building blocks and yet we are so diverse that no two individuals are alike even identical twins are not exactly identical, they grow up to become two separate individuals.
Sequencing human genome
As I said above, the entire book of life is written in four nucleotide base pairs, and they are A-T and G-C. A string of AT/GC base pairs is called the Deoxyribose Nucleic Acid (DNA). Out of four nucleotides, three nucleotides code for an amino acid called Codon. Different combination of four nucleotides gives sixty-four Codons which code for all twenty amino acids. The entire genome is written using six billion four hundred million nucleotides. Less than two percent of our genome carries genes which code for protein. Thousands of proteins interact to give a single cell; millions of cells interact to give a tissue; 220 different tissues interact to give an organ and several organs interact to make a human. In 1990, US Congress authorized three billion dollars to our Institute NIH (National Institutes of Health) to decipher the entire Human Genome under the title, “The Human Genome Project.” We found that our genome contains six billion four hundred million nucleotides base pairs and half comes from our father and another half comes from our mother. Less than two percent of our Genome contains genes which code for proteins. The other 98 percent of our genome contains switches, promoters, terminators, enhancers etc. The 46 chromosomes present in each cell of our body are the greatest library of the Human Book of Life on planet Earth. The chromosomes carry genes which are written in nucleotides. Before sequencing (determining the number and the order of the four nucleotides on a Chromosomes), it is essential to know how many genes are present on each chromosome in our Genome. The Human Genome Project has identified not only the number of nucleotides on each chromosome, but also the number of genes on each chromosome.
The following list provide the details composition of each chromosome including the number of nucleotides and the number of genes on each chromosome: We found that the Chromosome-1 is the largest Chromosome carrying 263 million A-T, and G-C nucleotides base pairs and it has only 2,610 genes. The Chromosome-2 contains 255 million nucleotides bases and has only 1,748 genes. The Chromosome-3 contains 214 million nucleotide bases and carries 1,381 genes. The Chromosome-4 contains 203 million nucleotide bases and carries 1,024 genes. The Chromosome-5 contains 194 million nucleotide bases and carries 1,190 genes. The Chromosome-6 contains 183 million nucleotide bases and carries 1,394 genes. The Chromosome-7 contains 171 million nucleotide bases and carries 1,378 genes. The Chromosome-8 contains 155 million nucleotide bases and carries 927 genes. The Chromosome-9 contains 145 million nucleotide bases and carries 1,076 genes. The Chromosome-10 contains 144 million nucleotide bases and carries 983 genes. The Chromosome-11 contains 144 million nucleotide bases and carries 1,692 genes. The Chromosome-12 contains 143 million nucleotide bases and carries 1,268 genes. The Chromosome-13 contains 114 million nucleotide bases and carries 496 genes. The Chromosome-14 contains 109 million nucleotide bases and carries 1,173 genes. The Chromosome-15 contains 106 million nucleotide bases and carries 906 genes. The Chromosome-16 contains 98 million nucleotide bases and carries 1,032 genes. The Chromosome-17 contains 92 million nucleotide bases and carries 1,394 genes. The Chromosome-18 contains 85 million nucleotide bases and carries 400 genes. The Chromosome-19 contains 67 million nucleotide bases and carries 1,592 genes. The Chromosome-20 contains 72 million nucleotide bases and carries 710 genes. The Chromosome-21 contains 50 million nucleotide bases and carries 337 genes. Chromosome-22 contains 56 million nucleotides and carries 701 genes. Finally, the sex chromosome of all females called the (X) contains 164 million nucleotide bases and carries 1,141 genes. The male sperm chromosome contains 59 million nucleotide bases and carries 255 genes.
If you add up all genes in the 23 pairs of chromosomes, they come up to 26,808 genes and yet we keep on mentioning 24,000 genes needed to keep us function normally. As I said above, a gene codes for a protein, not all 24,000 genes code for proteins. It is estimated that less than 19,000 genes code for protein. Because of the alternative splicing, each gene codes for more than one protein. All functional genes in our body make less than 50,000 protein which interact in millions of different ways to give a single cell. Millions of cells interact to give a tissue; hundreds of tissues interact to give an organ and several organs interact to make a human. Slightest damage to a single nucleotide in the coding region caused by either ionizing radiations, chemical/environmental pollutants, viral infections, genetic inheritance or by deletion, insertion, or inversion or relocation of DNA result in mutations. Mutated genes code for wrong proteins resulting in diseases [6]. Not all genes act simultaneously to make us function normally. Current studies show that a minimum of 2,000 genes are enough to keep human function normally; the remaining genes are backup support system, and they are used when needed. The non-functional genes are called the Pseudogenes. For example, millions of years ago, humans and dogs shared some of the same ancestral genes; we both carry the same olfactory genes, only in dogs they still function to smell searching for food. Since humans don’t use these genes to smell for searching food, these genes are broken and lost their functions, but we still carry them. We call them Pseudogenes. Recently, some Japanese scientists have activated the Pseudogenes, this work may create ethical problem in future as more and more Pseudogenes are activated. Nature has good reasons to shut off those Pseudogenes.
The post genomic era
More than 20 years have passed since we completed the Human Genome Project. By using Nanopore sequencer which sequence the genome cheaper and faster, we are sequencing the genome of many other species from microbes to mosquitoes, to monkeys and to men to study evolutionary changes occurred over eons. We have also sequenced the genomes of thousands of people. By comparing the genomes of healthy person, we are identifying variations called polymorphism. We found the answer to the question why no two people look alike. When we compare the genomes of two persons, we found that between 1,000 to 1,300 nucleotide base pairs, a single nucleotide base pair is located at a different place. This variation is called the Single Nucleotide Polymorphism (SNP). Our genome is made of three billion two hundred million nucleotide base pairs and carries three million two hundred million variants (SNP). These variants make us different from each other except in the identical twins. Comparing genomes of healthy person with a sick patient will help us identify the mutation responsible for causing the disease. For example, when we compare the nucleotides sequence of Chromosome-17 of two persons, if we find a mutation in the coding region, we detect the presence of a new amino acid. The peptide carrying the amino acid is responsible for causing mental retardation called the Fragile-X Syndrome. Another source of aging is the shortening of the telomeres. As I said above, the end of each chromosome, there is a six-letter code (TTAGGG) called the Telomere. Telomere are related to aging. In each white blood cells, the length of telomeres ranges from 8,000 base pairs in newborns to 3,000 base pairs in adults and as low as 1,500 in elderly people. Telomeres are shortened because of cellular replication, leading to a permanent cell cycle arrest, also known as replicative senescence. Research suggests that preserving telomeres length has the potential to prevent and treat diseases associated with aging and possibly allow humans to increase their longevity beyond the current theoretical maximum of 125 years. It is possible to slow down shortening of telomeres by maintaining a healthy weight with healthy eating, exercising regularly, quitting smoking, getting enough sleep, reducing, or managing stress, eating a telomereprotective diet full of foods high in vitamin C, and polyphenols.
As I said above, the number of telomeres in our bodies declines as we age. It is possible not only to maintains but also to lengthen the numbers of telomeres. There is scientific evidence that telomeres can be lengthened by using an enzyme called Telomerase Reverse Transcriptase (TRT). It can slow, stop, or perhaps even reverse the telomere shortening that happens as we age. If we prevent the loss of Telomeres by using the enzyme Telomerase Reverse Transcriptase (TRT), we could slow down the aging process. We have already demonstrated in the worm C. Elegance that we could increase its lifespan several fold. Now, we could translate this work in human being; we could try by making a less virulent Flu Virus, which could serve as Vector, carrying TRT gene when injected to a volunteer who comes down with a mild Flu. When he recovers from the Flu, the TRT gene would have inserted in the entire genome of every cell in his body (we can confirm the insertion by sequencing his genome). Suppose at each replication only half Telomeres are deleted instead. This person is likely to live twice as long. This work is likely to preserve human intelligence after the Sun dies as a super nova explosion. We must develop Plan B to save human life on some other Planet. We look up to Heaven to find another home for humanity. To search for a suitable Planet for human life to survive, we need to train an army of Astronauts to travel into deep space with extended life span. They may have to travel for centuries to find a habitable planet. We do not want them to die on their way to find a new home for humanity. We must continue to search for ways to prolong human life. Some simple-minded people, mostly religious people would say God created this wonderful Earth for us. Why can’t we live on Earth forever? Since God has created us, He will protect us. Science say that we cannot stay on Earth forever. We have limited time on Earth. If we want intelligent human life to survive, we must leave Earth. For deep space travel, we need to increase our life span beyond one hundred years
Treating Old Age Diseases to Slow Down Aging
Accumulation of mutations over years in autosomal chromosomes result in old age diseases. The aging process can be slowed down by developing novel drugs for treating old age diseases. The following are the old age diseases arranged in order of funding provided by NIH and they are Cancers, cardiovascular diseases, and Alzheimer. We can prolong human life if we find treatment of these diseases.
Cancers
Cancer is the leading cause of death and has surpassed the death of cardiovascular diseases. Over 636,000 people died of cancer; 1.9 million new cases will be diagnosed this year including 78,000 Prostate Cancer, 40,000 Breast cancer, 16,000 Lung and Bronchus Cancer and 15,000 Colon and Rectal Cancer. Once diagnosed by Gene Sequencing, the next step is to design drug to shut off those genes.
The rational drug design to treat cancers
All three old age diseases that is Cancer, Cardiovascular Diseases and Alzheimer carry multiple somatic mutated genes responsible for causing these diseases. In each of the above three diseases, it is the accumulation of the of the somatic mutations in genes, over decades in the first 22 pairs of autosomal chromosomes, which is responsible for coding the wrong protein which causes these diseases. If we design drugs to shut off mutated genes in one disease, using the same rationale, we should be able to shut off bad genes in all three old age diseases. Although Coronary Artery disease is a complex disease, researchers have found about 60 genomic variants that are present more frequently in people with Coronary Artery Disease. Most of these variants are dispersed across the genome and do not cluster on one specific Chromosome. Drugs are designed to seek out the specific malignant genes which replicate faster producing acids. Aziridines and Carbamate moieties serve as prodrugs and are sensitive to acid. Drugs carrying the Aziridines, and Carbamate moieties are broken down in acidic media generating highly reactive Carbonium ions which attack DNA shutting off genes. Only the acid producing genes will be attacked no matter where they are located. It does not matter whether they are clustered or dispersed across the genome. The supreme intellect for Drug Design is Ross, an Englishman, who is a Professor of Chemistry at the London University, England. Professor WCJ Ross is also the Head of Chemistry Department at the Royal Cancer Hospital, a post-graduate medical center of the London University. Ross was the first person who designed drugs for treating Cancers. He designed drugs to cross-link both strands of DNA that we inherit one strand from each parent. Cross-linking agents such as Nitrogen mustard are extremely toxic and were used as chemical weapon during the First World War (WWI). More toxic derivatives were developed during the Second World War (WWII). Using the Data for the toxic effect of Nitrogen Mustard used during the First World War, Ross observed that Soldiers exposed to Nitrogen Mustard showed a sharp decline of White Blood Cells (WBC) that is from 5,000 cell/CC to 500 cells/CC. Children suffering from Childhood Leukemia have a very high WBC count over 90,000 cells/CC. In sick children, most of the WBCs are premature, defected, and unable to defend the body from microbial infections. Ross rationale was that cancer cells divide faster than the normal cell, by using Nitrogen Mustard to cross linking both strands of DNA, one can control and stop the abnormal WBC cell division in Leukemia patients.
It was indeed found to be true. Professor Ross was the first person to synthesize many derivatives of Nitrogen Mustard. By using an analog of Nitrogen Mustard, called Chlorambucil, he was successful in treating Childhood Leukemia. In America, two Physicians named Goodman and Gilman from the Yale University were the first to use Nitrogen Mustard to treat cancer in humans. Nitrogen Mustards and its analogs are highly toxic. Ross was a Chemist, over the years, he synthesized several hundred derivatives of Nitrogen Mustard molecules to modify toxicity of Nitrogen Mustard [7-9]. Although analogs of Nitrogen Mustard are highly toxic, they are more toxic to cancer cells and more cancer cells are destroyed than the normal cells. Toxicity is measured as the Chemotherapeutic Index (CI) which is a ratio between toxicity to Cancer cells versus the toxicity to Normal cells. Higher CI means that the drugs are more toxic to cancer cell. Most cross-linking Nitrogen Mustard have a CI of 10 that is they are ten times more toxic to cancer cells. Some of the Nitrogen Mustard analogs Ross made over the years are useful for treating cancers such as Chlorambucil for treating childhood leukemia (which brought down the WBC level down to 5,000/CC). Children with Childhood Leukemia treated with Professor Ross Chlorambucil showed no sign of Leukemia even after 20 to 25 years. Chlorambucil made Ross one of the leaders of the scientific world. He also made Melphalan and Myrophine for treating Pharyngeal Carcinomas [10-13].
Figure 1:Design drugs to attack only one strand of DNA.
At the London University, I was trained as an Organic Chemist in the Laboratory of Professor WCJ Ross of the Royal Cancer Hospital, a post-graduate medical center of the London University. After completing my doctoral and postdoctoral work, I was offered a permanent position where I worked for about ten years. I moved to America when I was honored by the Fogarty International Fellowship Award by the National Institutes of Health, NIH, and the National Cancer Institute, NCI, of the USA. NIH has been my home for over a quarter of a century, I designed drugs to shut off mutated genes. All three old age diseases have Common genetic origin. The rationale I used to synthesize anti-cancer drugs could be used to treat the other two old age diseases like Alzheimer or Cardiovascular Diseases. In the following sections, I will describe in detail how anti-cancer drug like CB1954 was synthesized to treat animal tumor and the animal work was translated in human by making AZQ which was designed to shut off Glioblastoma genes to treat Brain Cancer in humans. Using the same rational, we will consider how each of the other two old age diseases namely cardiovascular disease and Alzheimer could be treated by shutting off their bad genes to save human life. The order to work on these diseases are arranged based on the level of funding provided by NIH specifically by the NCI (National Cancer Institute). As I said above, Professor Ross was designing drugs to attack both strands of DNA simultaneously by cross-linking using Nitrogen Mustard analogs, which are extremely toxic. As a part of my doctoral thesis, I was assigned a different path. Instead of cross-linking DNA, I am to design drugs to attack only one strand of DNA (Figure 1).
DNA Binding Aziridine Group
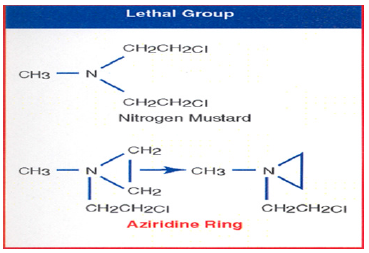
Figure 2:The above structures are Nitrogen Mustard (2-bischloroethyl methyl amine) and Aziridine.
Nitrogen Mustard neither have selectivity nor specificity. They attacked all dividing cells including normal cells. During the study of the mechanism of action of radiolabeled Nitrogen Mustard on DNA, it was discovered that the two arms of Nitrogen Mustard do not bind to the double stranded DNA simultaneously. It binds to one strand of DNA at a time. The carbonium ion of the other arm of Nitrogen mustard is so reactive it attacks its own Nitrogen atom forming a stable three-member aziridinium ion. We were unable to isolate the aziridinium ion as growing tumor which produces acid which break down aziridinium ion to produce a second carbonium ion which attacks the second strand of DNA. We were able to isolate cross-linking DNA product. This study showed that to attack a single strand of DNA, we must synthesize Aziridine analogs in the Lab. Synthesis of Aziridine analogs will give two advantages over Nitrogen Mustard: first, instead of cross-linking, Aziridine binds to one strand of DNA, reducing the toxicity of double strand Nitrogen Mustard by half. Second, it gives selectivity, the Aziridine ring opens only in the acidic medium. Once the active ingredient Aziridine was determined to attack DNA, the next question was what drug delivery method should be used to deliver Aziridine at the tumor site (Figure 2).
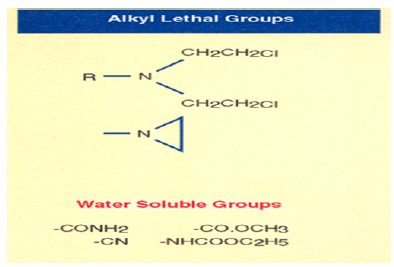
DNA Binding Lethal Groups
The novel class of cyclic three-member ring carrying Nitrogen is called Aziridines. Over the years, I made over 100 Dinitrophenyl Aziridines derivatives. One of them is Dinitro benzamide (CB1954) which gives a CI of 70 highest ever recorded. CB1954 wipes out a solid tumor by attacking the DNA of Walker Carcinoma 256, a solid aggressive tumor in Rat. As I said above, Nitrogen Mustards are highly toxic because they have neither specificity nor selectivity. They attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates serve as prodrug that is they remain inactive in the basic and neutral media. They become activated only in the presence of Acidic media. I used a simple rationale, the Aziridine attacks DNA in acidic medium, particularly the N-7 Guanine. The dye Dinitro benzamide has great affinity for Walker Tumor. The Aziridine dinitro benzamide (CB1954) stain the tumor. As the tumor grows, it uses Glucose as a source of energy. Glucose is broken down to Lactic Acid. It is the acid which attacks the Aziridine ring. The ring opens to generate a Carbonium ion which attacks the most negatively charged N-7 Guanine of DNA shutting off the Walker Carcinoma gene in Rat. To continue my work, I was honored with the Institute of Cancer Research Post-Doctoral Fellowship Award of the Royal Cancer Hospital of London University. To increase the toxicity of CB1954 to Walker Carcinoma, I made additional 20 analogs as a postdoctoral fellow. When I attached one more Carbonium generating moiety, the Carbamate moiety to the Aziridine Dinitrobenzene, the compound Aziridine Dinitro benzamide Carbamate was so toxic that its Therapeutic Index could not be measured. We stop the work at the London University for the safety concern. I continued my work on the highly toxic Aziridine/Carbamate combination in America when I was offered the Fogarty International Fellowship Award to continue my work at the National Cancer Institute (NCI) of the National Institutes of Health (NIH). I brought the idea from London University of attacking one strand of DNA using not only Aziridine, but also Carbamate without using the same dye Dinitro benzamide.
Designing drugs to bind to a single stranded DNA to treat animal cancers
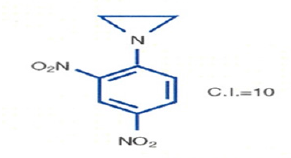
As a part of my doctoral thesis, I was assigned a different path. Instead of cross-linking DNA by Nitrogen Mustard, I am to design drugs to attack only one strand of DNA by making Aziridine analogues. We decided to use Aziridine moiety that would be an excellent active component to shut off a gene by binding to a single strand of DNA. To deliver Aziridine to the target site DNA, we decided to use Dinitrophenyl moiety as a delivery agent because its analog Dinitrophenol disrupt the Oxidative Phosphorylation of the ATP (Adenosine Triphosphate) which provides energy to perform all our body functions. To provide energy to our body function, the high energy phosphate bond in ATP is broken down to ADP (Adenosine Diphosphate) which is further broken down to AMP (Adenosine Mono Phosphate), the enzyme Phosphokinase put the inorganic phosphate group back on the AMP giving back the ATP. This cyclic process of Oxidative Phosphorylation is prevented by Dinitrophenol. I decided to use Dinitrophenol as drug delivery method for the active ingredient Aziridine. Dinitrophenol also serves as a dye which stains a tumor called the Walker Carcinoma 256, a solid and most aggressive tumor in Rat. The first molecule I made by attaching the C-14 radiolabeled Aziridine to the dinitrophenol dye. The Dinitrophenyl Aziridine was synthesized using Dinitrochlorobenzene with C-14 radiolabeled Aziridine in the presence of Triethyl amine which removes the Hydrochloric Acid produced during the reaction. When the compound Dinitrophenyl Aziridine was tested against the implanted experimental animal tumor, the Walker Carcinoma 256 in Rats, it showed a TI (Therapeutic Index) of ten. The TI was like most of the analogs of Nitrogen Mustard. Since this Aziridine analog was not superior to Nitrogen Mustard, it was dismissed as unimportant. Reexamination of the X-ray photographs showed that most of the radioactivity was concentrated at the injection site. Very little radioactivity was observed at the tumor site. It was obvious that we need to make derivatives of Dinitrophenyl Aziridine to move the drug from the injection site to the tumor site. Because of the lack of an effective drug delivery method, Dinitrophenyl Aziridine stays at the injection site. A very small amount of radioactivity was found on the tumor site (Figure 3).
Figure 3:Dinitrophenyl aziridine carrier of the lethal group.
Dinitrophenyl Benzamide a Novel Drug Delivery Molecule for Aziridine
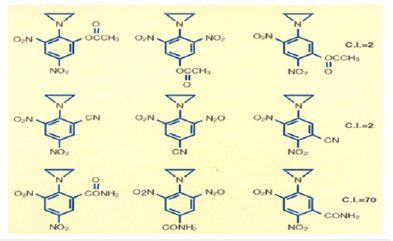
I immediately realized that by making water and fat-soluble analogs of Dinitrophenyl Aziridine, I should be able to move the drug from the injection site to the tumor site. To deliver 2,4-Dinitrophenylaziridine form the injection site to tumor site, I could alter the structure of 2,4-Dinitrophenylaziridine by introducing the most water-soluble group such as ethyl ester to least water-soluble group such as Cyano- group or to introduce an intermediate fat/water double Amido group. An additional substituent in the Dinitrophenyl Aziridine could give three isomers, Ortho, Meta, and Para substituent. Here confirmational chemistry plays an important role in drug delivery. Ortho substituent always give inactive drug. Model building showed that because of the steric hinderance, Aziridine could not bind to DNA shutting off the genes. On the other hand, Meta and Para substituents offer no steric hindrance and drug could be delivered to DNA. The following chart showed that I synthesized all nine C-14 radiolabeled analo of 2,4-Dinitrophenyl aziridines and tested them against implanted Walker Carcinoma 256 in Rats (Figure 4).
Figure 4:Most active drug against walker carcinoma in rat.
Derivatization of Dinitro Phenyl Benzamide based on Partition Coefficient
The most water-soluble substituents
The first three compounds on top line of the above chart carry all three isomer of most water-soluble Ethyl Ester group attached to 2,4-Dinitropehny aziridine. The compound in vivo is hydrolyzed ester to produce most water-soluble carboxylic group. Within 24 hours of injection, the entire radioactive compound was extracted from the Rat’s urine washed down from the cages. Since the Ortho position was not available for DNA binding, it showed no biological activity, but the third compound in which Ortho position was free to bind to DNA showed some activity.
The least water-soluble substituents
On the other hand, when the least water-soluble Cyano-group was attached to all three isomers of the 2,4-Dinitrophenyl aziridine compound as shown in the second line of the above chart, most of the compound stayed at the injection site. Only the last Cyanoderivative attached to DNA showed some activity.
The moderately soluble substituents
Figure 5:Conjugated DNA disrupting protein synthesis pathway of cancer cell.
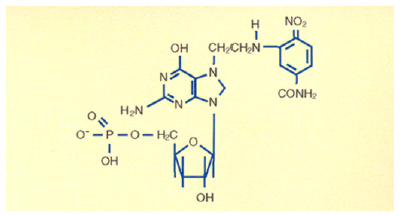
Figure 6:The Best and the worst dinitro phenyl aziridine analogs.
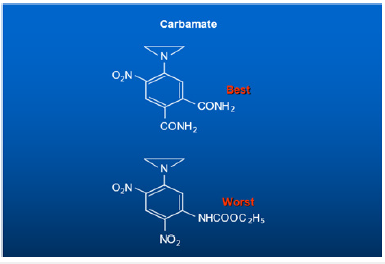
The last line of the above chart showed that the first two Amido groups were sterically hindered and did not bind to DNA and showed no biological activity, but the last compound presents the perfect drug delivery method. The entire drug was delivered from the injection site to the tumor site. The drug 1-Aziridine, 2,4-dinitro, 5-benzamide (CB1954) showed the highest biological activity. It has a CI of seventy; it is seventy times more toxic to cancer cells, highest toxicity ever recorded against Walker Carcinoma 256 in Rats [14-16]. Let me summarize: Nitrogen Mustards are highly toxic because they have neither specificity nor selectivity. They attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates serve as prodrug and remain inactive in the basic and neutral media. They become activated only in the presence of acid producing cancer cells. Aziridine attacks DNA in acidic medium, particularly the N-7 Guanine. The dye Dinitro benzamide has great affinity for Walker Tumor. The Aziridine Dinitro benzamide (CB1954) stain the tumor. As the tumor grows, it uses Glucose as a source of energy. Glucose is broken down to Lactic Acid. It is the acid which activates the Aziridine ring. The ring opens to generate a carbonium ion which attacks the most negatively charged N-7 Guanine of DNA shutting off the Walker Carcinoma gene in Rat. The following conjugate structure show how CB1954 binds to a single stranded of DNA shutting off the gene (Figure 5). For the discovery of CB1954, The University of London, honored with the Institute of Cancer Research (ICR) post-doctoral award to synthesize more analogs of CB1954. To improve drug delivery method, over the years, I made over a hundred additional analogs of Dinitro phenyl aziridine, one of them is aziridine dinitrophenyl Carbamate which was so toxic that its Therapeutic Index could not be measured. To increase the toxicity of CB1954 to Walker Carcinoma, I made additional 20 analogs as a postdoctoral fellow. When I attached one more Carbonium ion generating moiety, the Carbamate moiety to the Aziridine Dinitrobenzene, the compound Aziridine Dinitro benzamide Carbamate was so toxic that its Therapeutic Index could not be measured. We stop the work. Further work in London Unive was discontinued for safety reason. (Figure 6) I continued my work on the highly toxic Aziridine/Carbamate combination in America when I was offered the Fogarty International Fellowship Award to continue my work at the National Cancer Institute (NCI) of the National Institutes of Health (NIH). I brought the idea from London University of attacking one strand of DNA using not only Aziridine, but also Carbamate without using the same dye Dinitro benzamide.
Designing drugs to treat Glioblastoma, the human brain cancers
Figure 7:The structure of a non-toxic and nonaddictive quinone used for crossing the Blood Brain Barrier (BBB).
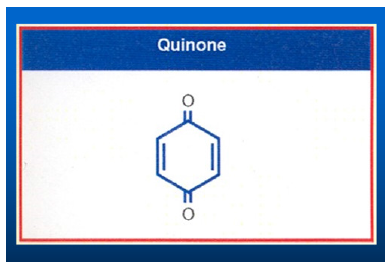
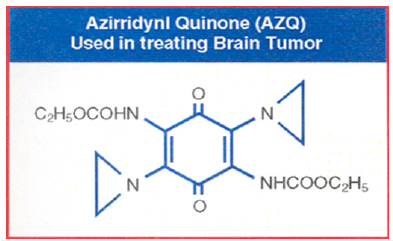
As I said above, my greatest challenge at NCI is to translate the animal work which I did in London University to humans. One day, I heard an afternoon lecture at the NIH in which the speaker described that radio labeled Methylated Quinone crosses the Blood Brain Barrier (BBB) in mice. When injected in mice, the X-ray photograph showed that the entire radioactivity Methylated Quinone was concentrated in the Mice’s brain within 24 hours. I immediately realized that Glioblastoma multiforme, the brain tumor in humans, is a solid aggressive tumor like Walker Carcinoma in Rats. I decided to use Quinone moiety as a novel drug delivery molecule to cross BBB delivering Aziridine rings to attack Glioblastomas. By introducing an additional Carbamate moiety, I could increase its toxicity several folds. I planned to use this rationale to translate animal work to human by introducing multiple Aziridine and Carbamate moieties to the Quinone to test against Glioblastomas in humans. Among all the derivatives, attaching two Aziridines and two Carbamate moieties to Quinone, I made the most active Quinone dicarbamate diaziridine compound. I named this novel compound AZQ. By treating brain cancer with AZQ, we observed that Glioblastoma tumor not only stop growing, but also start shrinking. I could take care of at least one form of deadliest old age disease that is Glioblastomas. Literature search showed that AZQ is extensively studied (Figure 7).
Glioblastoma (GBM) is a primary type of brain cancer which originates in the brain, rather than traveling to the brain from other parts of the body, such as the lungs or breasts. GBM is also called glioblastoma multiforme which is the most common type of primary brain cancer in adults. Attaching Nitrogen Mustard group to Quinone will produce highly toxic compound which will have neither specificity nor selectivity. Such a compound will attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates are prodrug and remain inactive in the basic and neutral media. They become activated only in the presence of acid producing cancer cells.
DNA binding aziridines
I continued my work on the highly toxic Aziridine/Carbamate combination in America. As I said, I brought the idea from London University of attacking one strand of DNA using not only Aziridine, but also Carbamate without using the same dye Dinitro benzamide. I decided to use Quinone moiety as a carrier for Aziridine rings to attack Glioblastomas. By introducing an additional Carbamate moiety, I could increase its toxicity several folds. Over the years, I made 45 analogs of AZQ. They were all considered valuable enough to be patented by the US Government (US Patent 4,233,215). By treating brain cancer with AZQ, we observed that Glioblastoma tumor not only stops growing, but it also starts shrinking. I could take care of at least one form of deadliest old age cancers, Glioblastomas. Literature search showed that AZQ is extensively studied [17,18]. As I said above, Glioblastomas, the brain cancers, is a solid and aggressive tumor and is caused by mutations on several chromosomal DNA. Deleterious mutations of DNA are the result of damaging DNA nucleotides by exposure to radiations, chemical and environmental pollution, viral infections, or genetic inheritance. The other factors responsible for causing DNA mutations are due to the fast rate of replication of DNA. For example, the bacteria E-coli grows so rapidly that within 24 hours, a single cell on a petri dish containing nutrients forms an entire colony of millions when incubated on the Agar Gel. Mistakes occur in DNA during rapidly replication such as Insertion of a piece of DNA, Deletion, Inversion, Multiple Copying, Homologous Recombination etc. When an additional piece of nucleotide is attached to a DNA string, it is called Insertion, or a piece of DNA is removed from the DNA string; it is called Deletion. In addition, translocation of DNA, or structural Inversion of DNA is also responsible for mutations. Since the gene in a DNA codes for Proteins, Insertion and Deletion on DNA have catastrophic effects on protein synthesis.
With the Quinone ring as a carrier across BBB, I could introduce different combinations of Aziridine rings and Carbamate moieties to Quinine and could create havoc for Glioblastomas. My major concern was how toxic these compounds would be to the human brain cells. Fortunately, brain cells do not divide, only cancer cells divide. Glioblastomas represent such an example. In Glioblastomas, three major changes occur on Chromosomes (C-7, C-9 & C-10) and two minor changes occur on Chromosomes (C-1 & C-19). These mutations are responsible for causing brain cancers in humans. In a normal human cell, Chromosome-7 which is made of 171 million nucleotide base pairs, and it carries 1,378 genes. When Insertion occurs on Chromosome-7. Ninety-seven percent of Glioblastoma patients are affected by this mutation. On the other hand, a different mutation occurs on Chromosome-9 which is made of 145 million nucleotide base pairs, and it carries 1,076 genes. A major Deletion of a piece of DNA occurs on Chromosome-9 which results in eightythree percent patients who are affected by this mutation. A minor Deletion of DNA also occurs on Chromosome-10 which is made of 144 million base pairs, and it carries 923 genes. Although it is a minor deletion of a piece of DNA and yet it contributes to ninetyone percent patients with Glioblastoma. To a lesser extent, small mutation occurs on Chromosome-1 (the largest Chromosome in our Genome; it is made of 263 million nucleotide base pairs and carries 2,610 genes) and Chromosome-19 (it is made of 67 million base pairs and carries 1,592 genes) is also implicated in some forms of Glioblastomas. All known Glioblastomas causing genes are located on five different chromosomes and carries a total of 9,579 genes (Figure 8). It appears impossible to design drugs to treat Glioblastomas since we don’t know which nucleotide on which gene and on which chromosome is responsible for causing the disease. We can easily identify the site by binding to DNA with C-14 radiolabeled studies, we can also confirm by comparing with the mega sequencing project.
Figure 8:Single strand DNA binding aziridine and carbamate.
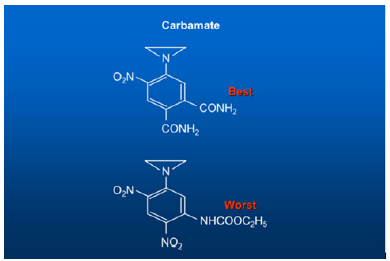
With the completion of 1,000 Human Genome Project, it becomes easier. By simply comparing the patient’s genome with the sequence of one thousand genomes, letter by letter, word by word and sentence by sentence, we could identify the difference called the mutated variants with precision and accuracy, the exact variants, or mutations responsible for causing the disease. Once the diagnosis is confirmed, the next step is how to treat the disease. As I said above, our Rational Drug Design work began in the University of London, England, and completed in the Laboratory of the National Cancer Institute (NCI), of the National Institutes of Health (NIH), in Bethesda, Maryland, USA. Over this period, I conducted over 500 experiments which resulted in 200 novel drugs. They were all tested against the experimental animal tumors. Forty-five of them were considered valuable enough to be patented by the US Government (US Patent 4,146,622). (Figure 9) One of them is AZQ. Radiolabeled studies showed that AZQ can cross organ after organ, cross the Blood Brain Barrier, cross the nuclear membrane, and attack the nuclear DNA shutting off the gene. X-ray studies showed that the radioactivity is concentrated in the tumor region. Glioblastoma stop growing and start shrinking. For the discovery of AZQ, I was honored with the “2004 NIH Scientific Achievement Award” one of America’s highest awards in medicine and I was also honored with the India’s National Medal of Honor, “Vidya Ratna” a Gold Medal. (Exhibits 1,2,3,4).
Figure 9:U.S. Patent 4,146,622.
2004 NIH Scientific Achievement Award Presented to Dr. Hameed Khan By Dr. Elias Zerhouni, The Director of NIH During the NIH/APAO Award Ceremony held on December 3, 2004.
Dr. Khan is the Discoverer of AZQ (US Patent 4,146,622), a Novel Experimental Drug Specifically Designed to shut off a Gene that causes Brain Cancer for which he receives a 17-year Royalty for his invention (License Number L-0I9-0I/0). To this date, more than 300 research papers have been published on AZQ. The award ceremony was broadcast live worldwide by the Voice of America (VOA). Dr. Khan is the first Indian to receive one of America’s highest awards in Medicine.
His Excellency, Dr. A.P.J. Abdul Kalam, The President of India Greeting Dr. A. Hameed Khan
Discoverer of anti-cancer AZQ, after receiving 2004, Vaidya Ratna, The Gold Medal, One Of India’s Highest Awards in Medicine. At The Rashtrapathi Bhavan (Presidential Palace), in Delhi, India, During a Reception held on April 2, 2004.
Gold Medal for Dr. Khan
Dr. A. Hameed Khan, a Scientist at the National Institutes of Health (NIH) USA, an American Scientist of Indian Origin was awarded on April 2, 2004. Vaidya Ratna; The gold Medal, one of India’s Highest Awards in Medicine for his Discovery of AZQ (US Patent 4,146,622) which is now undergoing Clinical Trials for Treating Bran Cancer
Cardiovascular diseases
Coronary Artery Disease is complex involving about 60 genomic variants (genes). All variants are not clustered on any specific Chromosome. These variants are dispersed across the entire genome. Although all variants have not been sequenced, we can shut off only the mutated gene without knowing the sequence of all other genes. As I mentioned above in the Cancer Section, the mutated gene grows rapidly forming the tumor. As it grows, it uses Glucose as a source of energy which is broken down to produce Lactic Acid. In the presence of Acid, the analogs of Aziridine and Carbamate are activated to generate Carbonium ion which attack the tumor DNA shutting off their genes. While we may someday be able to sequence all 60 genes associated with the Coronary Artery Disease, presently, we can single out and identify the mutated gene bound complex using radiolabeled Aziridine and Carbamate. The following example explain how some Arrhythmias causing genes could be identified and how drug could be designed to shut off these genes. The term “QT” refers to the segment of an electrocardiogram which measures the duration of time for the heart to relax after a heartbeat. In long QT syndrome, the duration of time is abnormally prolonged and creates a vulnerability to dangerous arrhythmias. Ever since the syndrome was described in 1957, researchers have engaged in a genetic race to identify the genes associated with long QT syndrome, which currently includes 17 genes. Three genes, KCNQ1, KCNH2 and SCN5A, had sufficient evidence to be implicated as “definitive” genetic causes for typical long QT syndrome. Four other genes had strong or definitive evidence supporting their role in causing atypical forms of long QT syndrome, presenting in the newborn symptoms associated with heart block, seizures, or delays in development. Once the mutated genes are identified, we could design drugs to shut off these genes as described in the Cancer Section.
Alzheimer
In 1906, the German Physician Scientist Dr. Alois Alzheimer identified the microscopic changes in the brain of a patient with the memory loss. He was the first Physician to identify the disease in a fifty-year old woman who suffered from Psychosis and who died within 4 years. Using special dyes, he stained the brain tissues which carried abnormal protein deposit around her brain which controlled brain function. He identified two kinds of legions of amyloid patches which he mistakenly thought was fatty patches and now turned out to be proteins. He observed a Patch of fatty deposit on the top of the brain cells called Plaques and the legions inside the nerve cells called Tangles. He accurately correlated the abnormal protein deposits around brain cells with the controlled of brain function. Today, we know that five million Americans and fifty five million people around the world suffer from Alzheimer and the Age is the single most risk factor for developing Alzheimer. By age 65 or older, the risk for developing Alzheimer is about 10 percent and by age 85 or older the risk factor is as high as 40 or 50 percent. As people grow old, they become senile. When he performed the autopsy of many senile persons, Dr. Alzheimer found the same Plaques and Tangles in many other samples. Early onset or late onset of Alzheimer resulted in the epidemic of Alzheimer. When comparing a normal brain with the Alzheimer brain, we find that the Alzheimer brain has shrunken and there is a concentration of Plaques and Tangles in neurons. In healthy brain cells, we see occasional Plaques and Tangles. It defines the disease; the Plaque and Tangles start building up as we grow old and over years and decades, the symptoms begin to develop. Symptoms include Memory loss and decrease ability of learning and recall. Early onset affects cognition which encompasses memory and other mental functions such as erosion of attention, thinking, reasoning, visual functions, spatial function, and Dementia with Memory loss and other cognitive functions resulting in mental impairment which affects to the degree interfering with the daily life.
Recent studies confirm that Alzheimer is an irreversible brain disorder which slowly destroys memory and thinking skills. The damage to the brain is not particularly associated to any specific gene, but the presence of the one form of the Apolipoprotein E (APOE) is a suspect gene whose presence does increase a patient’s risk for developing Alzheimer. The early onset of Alzheimer is associated with three single gene mutations: First, the presence of an Amyloid Precursor Protein (APP) located on Chromosome-21; the presence of Presenilin 1 (PSEN1) on Chromosome-14 and the presence of Presenilin 2 (PSEN2) located on Chromosome-1. All three Chromosomes are very large and carry hundreds of genes. For example, Chromosome-1 is the largest Chromosome in the Genome. It is made of 163 million nucleotide bases carrying 2,610 genes. Chromosome-21 is made of 50 million nucleotide bases carrying 337 genes while Chromosome-14 is made of 109 million nucleotides bases carrying 1,173 genes. A recent 7-million Utah population study identified two additional genes RAB10 located on Chromosome-2 (which is made of 155 million nucleotides bases and carry 1,798 genes) and SAR1A gene located on Chromosome-10 (which is made of 144 million nucleotide bases and carry 983 genes) associated with the formation of Plaques and Tangles. Mutations on these genes may be associated with the onset of Alzheimer
Of all the genes on these Chromosomes, only five single-gene mutations are associated with the early onset of the Alzheimer, it is the greatest challenge to design drugs to attack only the mutated genes. As I said above in the Cancer Section, the good news is that the only mutated genes grow rapidly using Glucose as a source of energy. Glucose is broken down to produce Lactic Acid. It is the acid which activate the Aziridine and Carbamate moieties producing powerful Carbonium ion which attack N-7 Guanine of DNA and shut off only the mutated genes. Other genes are not affected. Using C-14 radiolabeled Aziridines, we can identify the mutated gene which form the Aziridine/Protein Complex as described in the Cancer Section.
Rationale for Designing Drugs to Treat Alzheimer
It is well known that using the TFT dye, which is (3,6-dimethyl- 2-(4-dimethlaminophenyl)-benzothiazoline, could be used to stain the Plaques and Tangles of Alzheimer tissues. Using TFT dye as a carrier for the Aziridine and Carbamate moieties, we could design drugs to attack the mutated DNA to shut off genes which form Plaques and Tangles to prevent the progress of Alzheimer..
In the above Cancer section, I have described in detail how I had used Quinone as a carrier for Aziridine and Carbamate ions in designing AZQ to attack the brain tumor DNA to shut off genes for treating Brain Cancer. Similarly, the analogs of Benzothiazoline dyes could be used as carrier for Aziridine and Carbamate moieties to attack the genes responsible for producing Plaque and Tangle and prevent the development of Alzheimer.
What Other Cancers Should be Explored Next?
Of all cancers, the largest killer of women is the Breast Cancer. Despite the use of highly advanced treatment methods such as Chemotherapy, Radiation therapy and Surgery, within three years, the tumor returns as metastatic cancer and kill the patient. On the rational basis, I propose the following approach to develop novel drug to treat Breast Cancer. Although mutations on BRCA1 gene responsible for causing Breast Cancer located on Chromosome-17 has been identified years ago, so few drugs were designed on rational grounds. Now, we have sequenced Chromosome-17. We found that it is made of 92 million nucleotide bases pairs carrying 1,394 genes. By comparing with the Reference Sequence, we can easily identify which nucleotide on which gene of the Chromosome-17 is responsible for causing Breast Cancer. As I said above, Genomic medicine is a predictive medicine. By MRI (Magnetic Resonance Imaging which take three-dimensional X-ray images) and gene sequencing, we should be able to predict if the abnormal changes in the cellular DNA will lead to Breast Cancer. Without this knowledge, it has been so difficult to design drugs on rational basis to treat Breast Cancer. By the time the Breast Cancer diagnosis is confirmed in a patient, the BRCA1 gene has accumulated more than three thousand mutations. Genotyping of the blood sample would also show the existence of many cells carrying mutated cells responsible for creating secondary deposits. It is also found in some cases when not detected earlier, by the time Breast Cancer is confirmed, metastatic cancer cells have already been spread from Liver Lung on their way to Brain.
As a Fogarty International Postdoctoral Fellow at the NCI, I was given the chance to work on any cancer, I was pleased. Since all other organs including Breast and Liver could be removed and replaced by organ transplant except Brain, I thought that protecting Brain is utmost important to save life. For years, I work on the development of AZQ. Once the AZQ was developed to protect the Brain Cancer, I could focus on the Breast and Prostate Cancers. Recent, Radiolabeled studies in mice showed that male hormone Testosterone has great affinity for female organs like Breast, Ovary, and Fallopian tube cells. On the other hand, Estrogen, the female hormone, has great affinity for male Prostate gland. By attaching multiple Aziridine rings and Carbamate ions to both Hormones, I could design novel drugs to attack both the Breast and the Prostate cancers. Now, I found that I could increase its toxicity several folds to abnormal cells by attaching more than four Aziridine and Carbamate moieties to both Male and Female Hormones. In a Breast tumor, within the start and stop codon, BRCA1 gene has captured over two hundred thousand nucleotide bases. The BRCA1 gene carries about three thousand mutations. These mutations are caused by exposure to radiations, chemical or environmental pollutants, viral infection, or genetic inheritance. To attack the mutated nucleotides among the three thousand mutations in BRCA1 gene, we could use male hormone, Testosterone, and attach multiple radio labeled Aziridine and Carbamate ions to attack BRCA1 mutations. By using three dimensional MRI, we could show how many radio-labeled nucleotides were bound to which mutations. Out of seventeen positions available for substitutions on Testosterone ring system. There are only three positions that is 1,3 and 17 are available for substitution on Testosterone ring system.
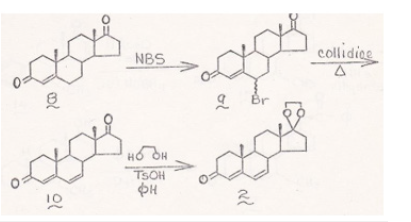
Carl Djerassi [C. Djerassi et al. J Amer Chem Soc 72: 4534 (1950)] had demonstrated that we could activate additional positions for substitutions on hormone ring system such as the position 9 and 10 by reacting with Bromo-acetamide which introduce a Bromo ion on position 10 which could be de-brominated by Collidine to introduce a 9,10 double bond which we could further brominate to produce 9,10 dibromo compound. (Figure 10) These bromo ion could be replaced by additional Aziridines or Carbamate ions. We could increase or decrease the number of Aziridine and Carbamate ions to get maximum benefit by further brominating position 15 and 16 to introduce additional Aziridine and Carbamate moieties. Similarly, we could use the female hormone Estrogen as a carrier by attaching multiple Aziridine and Carbamate ions to attack Prostate tumor in Men. Since there are seventeen positions also available on Estrogen ring as well; again, we could increase or decrease the number of Aziridine and Carbamate ions to get the maximum benefit by using Djerassi’ method as we used with Testosterone. The above methods are novel approach to designing drugs to treat Breast and Prostate cancers using genetic make-up of a patient to treat metastatic cancers.
Figure 10:Testosterone ring system.
Future challenges
The greatest challenge for the humanity is to survive before
our sun dies as a super nova. The following additional challenges
threatens our extinction: We have enough time
I. To Escape Earth only if we don’t destroy ourselves by
going to nuclear war. Our sun still has enough energy to help us
escape Earth. By working not only
II. To Prolong human life from centenarian to super
centenarian for deep space travel
III. To develop Hydrogen fusion reaction, to produce
unlimited clean energy, as it is taking place on the surface of
sun. Universe is filled with icy comets which can provide not
only water, but also Hydrogen as a fuel and lifesaving Oxygen.
IV. To gain enormous speed in space. There is no air above 33
miles; we need new generations of spacecrafts to travel faster
in vacuum. If we were to reach on one of the nearest exoplanets
V. To travel in space at least half the speed of light. The
development of SpaceX’ Starship is the best step in the right
directions. It is a reusable large-scale spacecraft. With more
than 5 million pounds of thrust at liftoff, it is more powerful
than Saturn V which sent men to the Moon. The Starship can lift
one hundred metric ton of payload at a time. Since the rocket
is reusable, we can ferry all Oxygen and Methane fuel to store
in the mother ship located in the near-Earth orbit. We can also
ferry all heavy equipment that will be built on Earth and will
be transported to space Lab to construct city-size spaceships in
the near-Earth orbit.
Our biggest challenge is to produce unlimited amount of safe energy by fusion reaction as it is taking place on the surface of Sun. The main fuels used in nuclear fusion are deuterium and tritium, both heavy isotopes of hydrogen. Fusion occurs when the mixture of these gases is heated to 150 million degree Celsius resulting in fusion of these gases to Helium and releasing the subatomic particles as unlimited amount of clean energy. Fusion reaction plants are expected to be operational by 2030. The most important information we need to know if there is life in our solar system. For example, is there is a microbial life Mars? What kind of life it is? And what it is made of? Is it a carbon-based life like on Earth? Unfortunately, the Rover Perseverance on the surface of Mars is not equipped with a DNA sequencer to sequence the Martian life. In future, all rovers designed to land on any celestial body must be equipped with the next generation Nanopore sequencer to sequence any piece of DNA and send the data back to Earth with the speed of light for analysis. If human intelligence is to survive in the cosmos after the destruction of our solar system, we must conquer Aging for deep space travel. Of all the creatures on Earth, humans are the most intelligent. It has taken three and a half billion years of biological evolution for the life on Earth to achieve this level of intelligence. If we don’t protect the human intelligence and let it parish with Earth, no one in the entire Cosmos will ever know, that at some remote corner of a Milky Way galaxy around its third spiral arm, there existed a middle age star, the Sun, and on its third orbit, there was planet Earth on it the human intelligent life ever existed.
Conclusion
Sooner or later, it would be genetically possible to slow down Aging. Prolonging human life may also create some serious ethical problems. By using genetic engineering methods, suppose we could slow down Aging by slowing the loss of Telomeres. For example, to prevent the loss of Telomeres, we can create a harmless virus (serve as vector) carrying Telomerase Reverse Transcriptase (TRT)
gene to infect human genome to insert the TRT gene and to prolong human life. Also suppose the method is inexpensive, safe, and easily available, should we prolong human life of all who wants to live long? Knowing full well that there are almost eight billion people live on Earth and we are adding 90 new individual each year. Should we prolong the life of everyone who wants it? Despite such threats as population expulsion, environmental collapse, or a nuclear war, must we continue to search for ways to prolong human life. Some simple-minded people, mostly religious people, would say yes; since God created this wonderful Earth for us, He alone would take care of us. Why can’t we live on Earth forever? Science say that we cannot stay on Earth forever. We have limited time on Earth. If we want intelligent human life to survive, we must prolong Age to leave Earth. Science also tells us that all evidence points to the death of our solar system as a super nova. Our Sun has been burning for the past four and a half billion years. It burns 700 million tons of Hydrogen every second. During its four and a half billion years of existence, it has used up more than half of its energy. Humanity is trapped in a middle age dying Solar System. We have a choice either to stay on Earth and die with it or to get out of this Solar System and survive. If you are religious person, you will leave your fate in the hands of God and go to Heaven. If you believe in Science, you will plan to escape Earth before it dies. Humanity has come to a crossroad; where our path bifurcates; one path leads to total inhalation and destruction of all life forms on Earth and the other path leads to escape from Earth to live to discover new worlds before the Earth with all life forms turn to ashes and become a part of the big cosmos. We came from stardust, and we end up as stardust. We have a choice either to learn to prolong human life to escape Earth or parish with it. We hope that our leaders have enough wisdom to choose the right path. We must neither given in nor give up to the dying Solar System, we must escape. To succeed in the deep space travel before the end of this century, we need outstanding men and women from the citizens of the world. Who among you would be the vanguard of research and technology to spread human intelligence in the cosmos? We bequeath the future of humanity in your hands, we know you will do your best to protect, preserve and spread human intelligence in every corner of the Universe. As Sir Winston Churchill had said during WWII, “if we fail, we all fail and if we die, we all will die together.” The next generation of scientists (my students and their students) will ensure that humanity escapes Earth before it dies [19-42].References
- Watson JD, Crick FHC (1953) Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 171: 737-738.
- Hameed Khan (2001) The impact of sequencing human genome on genomic food & medicine. International Journal of Genetics and Genomics 9(1): 6-19.
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409(6822): 860-921.
- International Human Genome Sequencing Consortium (2004) Finishing the euchromatic sequence of the human genome. Nature 431(7011): 931-945.
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438: 803-810.
- Shendure J, Balasubramanian S, Church GM, Gilbert W, Rogers J, et al. (2017) DNA sequencing at 40: Past, present and future. Nature 550(7676): 345-353.
- Antonio MD, McLuckie KIE, Balasubramanian S (2014) Reprogramming the mechanism of action of chlorambucil by coupling to a G-Quadruplex ligand. Journal of the American Chemical Society 136(16): 5860-5863.
- Ross WCJ (1953) The chemistry of cytotoxic alkylating agents. Advances in Cancer Research 1:397-449.
- Ross WCJ (1962) Biological Alkylating Agents. London, UK.
- Ross WCJ (1949) Aryl-2-halogenoalkylamines. Part I. Journal of Chemical Society, pp. 183-191.
- Ross WCJ (1950) The reactions of certain epoxides in aqueous solutions. J Chem Soc, pp. 2257-2272.
- Ross WCJ, Mitchley BCV (1964) Ann Rep Brit Empire Cancer Campn. 42: 70.
- Thierry Facon, Yves Mary J, Cyrille Hulin, Lotfi Benboubker, Michel Attal, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Randomized Controlled Trial 370(9594): 1209-1218.
- Cobb LM, Connors TA, Elson LA, Khan AH, Mitchley BCV, et al. (1969) 2,4-dinitro-5-ethyleneiminobenzamide (CB 1954): a potent and selective inhibitor of the growth of the Walker carcinoma 256. Biochem Pharmacol 18(6):1519-1527.
- Khan AH, Ross WCJ (1969) Tumour-growth inhibitory nitrophenylaziridines and related compounds: Structure-activity relationships I. Chem Biol Interactions 1(1): 27-47.
- Khan AH, Ross WCJ (1971) Tumour-growth inhibitory nitrophenylaziridines and related compounds: Structure-activity relationships II. Chemico-Biological Interactions 4(1): 11-22.
- Khan AH, Driscoll JS (1976) Potential central nervous system antitumor agents: Aziridinylbenzoquinones I. Journal of Medicinal Chemistry 19(2): 313-317.
- Chou F, Khan AH, Driscoll J (1976) Potential central nervous system antitumor agents: Aziridinylbenzoquinones. Journal of Medicinal Chemistry 19(11): 1302-1308.
- Khan AH (2021) The impact of diagnostic MRI on the early detection of lethal genes in human genome and to develop genomic medicine to treat brain cancers. J Med Clin Res & Rev 5(6): 1-9.
- (2018 ) Development of oral cancer-Risk factors and prevention strategies. In: Moustafa AE (Ed.), Springer, USA.
- Khan AH (2021) The impact of diagnostic MRI on the early detection of lethal genes in human genome and to develop genomic medicine to treat brain cancers. J Med Clin Res & Rev 5(6): 1-9.
- (2021) The impact of sequencing human genome on genomic medicine and the discovery of AZQ.
- Specifically Designed to shut off genes that cause Brain Cancer. In: Berhardt LV (Ed.), Advances in Medicine and Biology, NOVA Medicine and Health 180(1): 1-63.
- Khan Ah (2021) The rational drug design to treat cancers. In: Parikesit AA (Ed.), Drug design- novel advances in the omics field and applications London, pp. 117.
- Khan H (2021) The impact of sequencing human genome on genomic food & medicine. International Journal of Genetics and Genomics 9(1): 6-19.
- Khan H (2021) The impact of sequencing genomes on the human longevity project. J Med Clin Res & Rev 5(7): 1-12.
- Hameed AK (2021) The impact of diagnostic MRI on the early detection of lethal genes in human genome and to develop genomic medicine to treat brain cancers. J Med Clin Res & Rev 5(6): 1-9.
- Khan H (2021) The impact of sequencing genomes on the human longevity project. J Med Clin Res & Rev 5(7): 1-12.
- Khan H (2021) The impact of sequencing human genome on genomic food & medicine. International Journal of Genetics and Genomics 9(1): 6-19.
- Khan H (2021) The rational drug design to treat cancers. In: Parikesit AA (Ed.), Drug Design - Novel Advances in the Omics Field and Applications, pp. 95-115
- EAS J Biotechnology Genet. In: Selvan TK (Ed.), East African Scholars Publisher, Kenya.
- Genomic medicine: Using genetic make-up of the human genome, AZQ was designed to treat glioblastoma, the brain tumor. Crimson Publishers AZQ was Designed to Treat Glioblastoma, Nov Appro in Can Study 6(2).
- Novel Approaches in Cancer Study 592 593 Nov Appro in Can Study Copyright © Hameed Khan NACS.000634. 6(2).2021
- Journal of Cancer Research Reviews & Reports- The Impact of Sequencing Human Genome on Cancer Chemotherapy
- Journal of Cancer Research Reviews & Reports: The Impact of Sequencing Human Genome on Cancer Chemotherapy J Can Res Rev Rep, 2021
- Khan AH (2021) The impact of sequencing human genome on cancer chemotherapy. Journal of Cancer Research Reviews & Reports 3(3): 1-10.
- Khan AH (2021) The impact of sequencing genomes on the understanding of the origin of life on earth. Biomedical J Sci & Tech Res 40(2): 32008-32022.
- Khan AH (2022) The impact of sequencing human genome on the genetically engineered life. Cancer Research and Cellular Therapeutics 6(1).
- Khan AH (2022) Sequencing the human genome to study the correlation between genes and violence. Horizon publishers.
- Khan AH (2022) The impact of sequencing human genome on the 2nd genesis on earth. Journal of Current Trends in Agriculture, Environment and Sustainability 3(1): 1-18.
- Khan AH (2022) Assessment of human genome sequencing on the new-world order. Research Aspects in Biological Science 4(6).
- Khan AH (2022) The impact of sequencing human genome on novel drug delivery method to treat cancers. International Journal of Genetics and Genomics 10(3): 64-78.
© 2022 Hameed Khan A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






