- Submissions

Full Text
Novel Research in Sciences
Dysfunction of the Glymphatic System is the Cause of Amyloid Angiopathy-Strong Evidence from a Case and Review of the Literature
Forshing Lui1*, Ning Zhong2, Kaho Wong3, Stella Knowlton1, Jessa Alcaide1 and Michael Ysit1
1California North state University College of Medicine, Elk Grove, California
2Kaiser Permanente Medical Center, Sacramento, California
3Kaiser Permanente Medical Center, Roseville, California
*Corresponding author: Forshing Lui MD, California North state University, College of Medicine, California
Submission: August 02, 2022;Published: August 25, 2022
.jpg)
Volume11 Issue5August , 2022
Abstract
There are no lymphatics within the brain like other organs in our body with traditional anatomical understanding until Iliff and Nedergaard demonstrated a system of cerebrospinal and brain interstitial fluid flow systems that appears to be like the lymphatic system in rodents. They created the term “glymphatic”. More recent studies by using MRI tracer studies in humans demonstrated evidence of a similar glymphatic system in humans. It is logical to believe that such a system functions as an important waste clearance system in health and disease. However, the function of such a system has been questioned by more recent studies. There has never been any real human case to confirm the pathophysiologically the dysfunction of such a system as the reason for the underlying disease. Furthermore, there has never been any explanation how beta-amyloid synthesized in the brain can be transported retrogradely to the small brain arteries and arterioles in amyloid angiopathy. We report a case of relatively rapid development of amyloid angiopathy over a few years after an acute subarachnoid hemorrhage secondary to rupture of the posterior communicating aneurysm. The amyloid angiopathy is relatively localized over the site of subarachnoid hemorrhage. There are also associated local changes and amyloid deposition in the brain parenchyma. We believe this case evidently and adequately demonstrated the function and dysfunction of the glymphatic system in waste clearance and movement of solutes in the brain parenchyma. It also demonstrated how a-beta amyloid is synthesized in the brain parenchyma and transported retrogradely towards and got deposited in the small cerebral arteries in amyloid angiopathy.
Keywords: Glymphatic; A-beta amyloid; Amyloid angiopathy; Proteinopathy
Abbreviations: CSF: Cerebrospinal Fluid, ISF: Interstitial Fluid, AD: Alzheimer’s Disease, Aβ: A-beta, AQP4: Aquaporin-4, MOCA: Montreal Cognitive Assessment Test, CAA: Cerebral Amyloid AngiopathyrIntroduction
The existence of a brain lymphatic system similar to systemic lymphatics is unknown to most neurologists and many neuroscientists. The present day, medical and neuroscience students learn the traditional concept of brain CSF production by the choroid plexus, circulating through the 4 ventricles and the subarachnoid space and returning to the systemic circulation through the arachnoid granulations. The modern understanding of the CSF flow dynamics has been revolutionized by the work done by Iliff and Nedergaard et al. [1,2] on rodents. They created the term “glymphatic” to describe the flow of Cerebrospinal Fluid (CSF) from the perivascular space (Virchow Robbin, VR space) accompanying the small cortical arteries or arterioles into the brain interstitial fluid (ISF) and drained out of the system into the deep cervical lymphatics. The efflux of CSF into the brain interstitial fluid depends heavily on the function and integrity of the astroglial water channels (Aquaporin-4, AQP4). Impaired CSF-ISF movement was demonstrated in AQP4 knock-out mice [3]. More recent human studies by using MRI with CSF tracer [4-8] demonstrated a similar system and its flow pattern in normal and some disease states. Taoka and Naganawa used the term “CNS interstitial fluidopathy” to define neurological disorders caused by impaired CNS interstitial fluid dynamics. It was also postulated that the water channels (AQP4) in the astroglial foot processes play an important part in the exchange of fluid and solutes between the CSF and the ISF. The glymphatic system provides an important waste clearance system for the brain to remove large molecular solutes and wastes such as amyloid beta (Aβ) and tau proteins. Impairment of this clearance system may play an important part in the pathogenesis of various neurodegenerative disorders including Alzheimer’s disease, agerelated changes, idiopathic normal pressure hydrocephalus, and traumatic brain injury [4,9]. However, different viewpoints arose recently questioning the significance of this glymphatic clearance system [10,11].
The Case
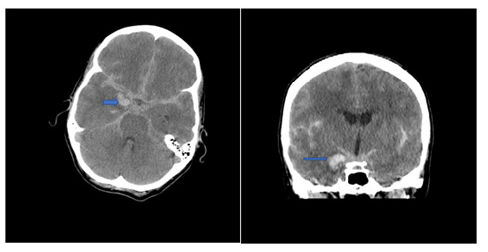
Figure 1: Acute SAH due to ruptured R PCoM aneurysm(arrow) (February 2016).
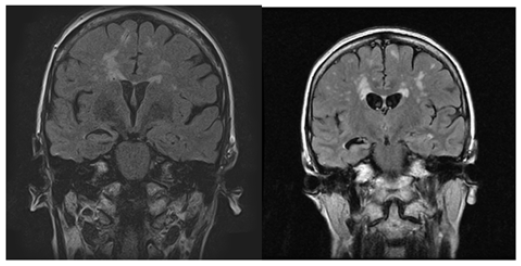
A. Severe progressive atrophy of her right hippocampus with relatively normal size of her left hippocampus (Figure 2).
Figure 2:MRI – FLAIR sequence: Progressive R hippocampal atrophy (August 2018) and (March 2021).
B. Progressive development of microhemorrhages as shown in serial GRE/SWI MRI. These are lobar microhemorrhages sparing deep hemisphere structures, most compatible with probable amyloid angiopathy according to the modified Boston Criteria. The diagnosis is further supported by the positive Amyloid PET scan (Figures 3 & 4).
Figure 3:Progressive development of R>L hemisphere amyloid angiopathy (GRE and SWI sequences).
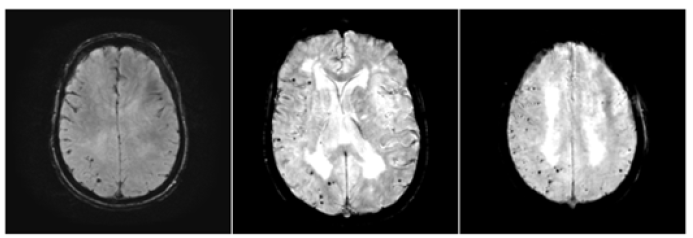
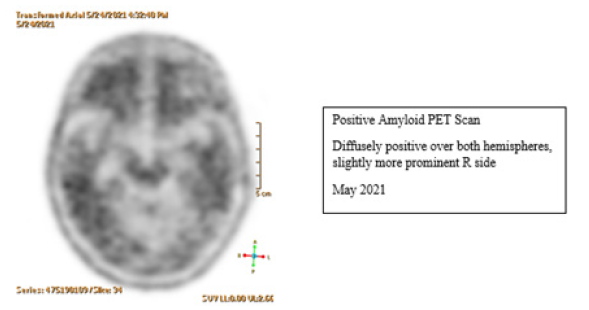
Figure 4:Diffusely positive amyloid deposition in Amyloid PET Scan: more prominent on the R hemisphere.
C. The microhemorrhages increased in number from 2018 to
2021. The number is consistently and significantly higher
affecting her right cerebral hemisphere (Figure 3).
D. Her recent amyloid PET scan showed diffuse cerebral amyloid
deposition, slightly more on her right hemisphere (Figure 4).
Our case is unique and there has not been any similar
case reported in the literature. It illustrated several important
clinicopathological changes which may help in the understanding of
neurodegenerative disorders because of impaired waste (protein)
clearance from the brain as follows:
a) The patient was neurologically normal clinically before she
suffered from an acute subarachnoid hemorrhage secondary to
rupture of a right posterior communicating artery aneurysm.
She might already have some early Alzheimer’s disease
pathology as evidenced by the widespread and diffuse amyloid
deposition demonstrated in her recent amyloid PET study.
b) She developed apparent progressive clinicopathological
changes in her brain after her acute subarachnoid
hemorrhage. These include progressive cognitive impairment,
progressive right hemisphere worse than left hemisphere
amyloid angiopathy (modified Boston criteria), progressive
right hippocampal atrophy. These can all be explained by
dysfunction of the glymphatic clearance system because of the
subarachnoid hemorrhage as discussed below.
Discussion
Amyloid beta (Aβ) peptide accumulation in the brain is one of the two main pathological hallmarks of Alzheimer’s Disease (AD). Aβ is deposited in extracellular neurites in the brain forming amyloid neuritic plaques, especially in areas including the temporoparietal neocortex and the hippocampus associated with early atrophy of the hippocampus in patients with AD. Hardy and Higgins reviewed molecular and biological evidence in molecular genetics suggesting Aβ is central to the etiology of AD [12,13]. The other pathological hallmark is the accumulation of hyperphosphorylated tau (pTau) protein inside the neurons as neurofibrillary tangles. Pathological Aβ is produced by sequential proteolytic cleavage of the transmembrane protein, Amyloid Precursor Protein (APP) by β- and γ-secretase enzymes [14]. [12] postulated the amyloid cascade hypothesis in 1992 in the pathogenetic mechanism of AD. Subsequent studies, however, failed to show the correlation between plague load and cognitive decline in patients with AD [15]. Nevertheless, the presence of Aβ neuritic plagues is still the most important pathological hallmark to define the disease. Imaging of Aβ such as amyloid PET scan is also used to identify underlying brain pathology as AD in patients with dementia or in clinical research.
The main pathological substrates in the brain and brain arteries are Aβ1-42 predominantly in neocortical and hippocampal neurites and the smaller and more soluble Aβ1-40 in the arterial walls of small Cerebral Arteries and Arterioles as Amyloid Angiopathy (CAA) [16]. It is interesting to note that CAA is uncommon in deep hemispheric structures including basal ganglia, thalamus, and brainstem. It is easily understandable that excessive synthesis of Aβ1-42 will lead to deposition in neurites especially in genetic and familial forms of AD such as cases due to presenilin-1 mutation, Down’s syndrome, and Apolipoprotein E4 genotypes. It will be difficult to visualize how Aβ1-40 which is also synthesized in the brain, can be “transported” retrogradely to the cerebral arteries and deposited in the walls of these arteries and arterioles resulting in subsequent complications of hemorrhage and ischemia. In the detailed review of Aβ17, Chen et al mentioned its clearance across the Blood-Brain Barrier (BBB) as one of the mechanisms of removal or clearance. It will be easier to understand its clearance down the pressure gradient towards the venous system than on the pressure gradient towards the arterial system [17]. Our case illustrates many unanswered questions about Aβ clearance and its increased deposition inside the cerebral neocortex and small arteries and arterioles. First, we need to review a more recently discovered cerebral waste clearance system involving the Cerebrospinal Fluid (CSF) and brain Interstitial Fluid (ISF) called the “glymphatics” system.
There is about 150 mL of CSF in an average adult. The 24 hours turnover is about 500 mL. The main known function of the CSF is to provide buoyancy for our brain. It is easily understood that there should be more important functions to explain the high CSF turnover. The logical reason should include metabolic supply and waste clearance for our metabolically active brain. The traditional view begins with its formation by the choroid plexus. It circulates through the 4 ventricles and is finally reabsorbed by the subarachnoid granulations. This concept has been challenged more recently both in CSF production and CSF removal [18,19]. For CSF production, a more recent concept supported by good scientific evidence is that a large volume of CSF is produced directly from the capillaries in the brain secondary to hydrostatic pressure, rather than strictly from the choroid plexus [20,21]. The removal of CSF solely by arachnoid granulations has been under stronger scrutiny by many studies over the past decade or so. Tracer studies of CSF flow [22,23] showed drainage through the lymphatics of the cribriform plate, cranial, and spinal nerve sheaths. The most significant contribution to our modern understanding of CSF drainage and brain waste clearance, and the existence of a brain structure like systemic lymphatics is the concept of “glymphatics” [24]. By using fluorescent CSF tracers on rodents, Iliff et al. were able to demonstrate a large volume of CSF in the subarachnoid space rapidly entering the brain parenchyma along the perivascular spaces (Virchow-Robin or VR space) of the cortical penetrating arteries. The perivascular CSF space and the brain interstitial fluid (ISF) space are separated by the foot processes of astrocytes which have abundant water channels (aquaporin 4, AQP4), which are polarized and allow only unidirectional movement of fluid. At this point, the CSF in the Virchow-Robin space was driven out to the brain ISF space by arterial pulsation and convective bulk flow. This CSF influx occurs diffusely and uniformly throughout all cortical deep penetrating arteries centripetally towards the perivenous spaces of the deep draining veins with drainage more restricted towards several large draining veins. Fluorescent tracers from deep nuclear structures such as the striatum and thalami are drained medially to the perivenous spaces of the great vein of Galen and the internal cerebral veins. The CSF-ISF flow enters the perivenous space again through the AQP4 channels and is finally drained from the brain through the deep cervical lymphatics.
More recent studies [10,11,23,25,26] by using MRI with gadobutrol CSF tracer demonstrated the glymphatic system in humans quite convincingly. Intrathecally administered CSF tracer enters the brain from cortical surfaces alongside the periarterial spaces and moves centripetally towards the deep structures. Clearance of tracer is delayed in aging patients and patients with dementia and patients with normal pressure hydrocephalus [27,28]. The glymphatic CSF and ISF flow alters with different physiological and pathological conditions. Natural sleep is associated with enhanced glymphatic flow and clearance [29,30]. Impaired glymphatic flow is shown in old age, in idiopathic normal pressure hydrocephalus, and traumatic brain injury [27,28]. The prevalence of CAA increases with age. Associations between CAA APOE4 genotype and Alzheimer’s Disease Neuropathological Changes (ADNC) has been documented with multiple studies [31- 33]. 78-98% of patients with ADNC were estimated to have CAA. On the other hand, only 25% of patients with CAA have ADNC. CAA is caused by deposition of the more soluble Aβ1-40 whereas amyloid plaque consists mainly of the less soluble Aβ1-42. Aβ1-42 is synthesized inside the brain from the more soluble oligomers with the pleated sheet structure in neuritic plaques. Pathological condition related, as in Alzheimer’s disease, or age-related increase in Aβ production in the brain is considered the cause of the deposition in the neurites or cerebral arteries. The Aβ1-40 is also produced in the brain. How it ends up in the cerebral arteries retrogradely with the CSF-ISF flow has not been studied. With the understanding of the CSF-ISF glymphatic flow system, there will be Aβ1-42 deposition if it is not adequately cleared from the brain. Our case demonstrated an impairment of glymphatic flow on the arterial side due to fibrin deposition and other mechanisms because of her acute subarachnoid hemorrhage. The AQP4 channels are expected to be damaged, and the polarity of flow may also be altered. In addition, the centripetal glymphatic flow from the cortical VR space to the deep venous perivenous spaces may be altered from a fluid dynamics standpoint. If there is impaired glymphatic inflow from the cortical areas affected by pathological changes in the VR CSF space and astroglial AQP4 channels secondary to the acute subarachnoid hemorrhage, the CSF-ISF may be directed towards these areas. When the polarity of the AQP4 channels is damaged, it will be easy to understand how the Aβ1-40 may be carried along with the ISF in a retrograde manner towards the cerebral arteries and becomes deposited in the arterial wall with the consequences (Figure 5).
Figure 5:Bulk CSF-ISF flow in normal and abnormal in our patient after her acute aneurysmal subarachnoid hemorrhage: amyloid AB42 deposition in neurites and AB40 in cerebral arteries-dysfunction of the glymphatic system.
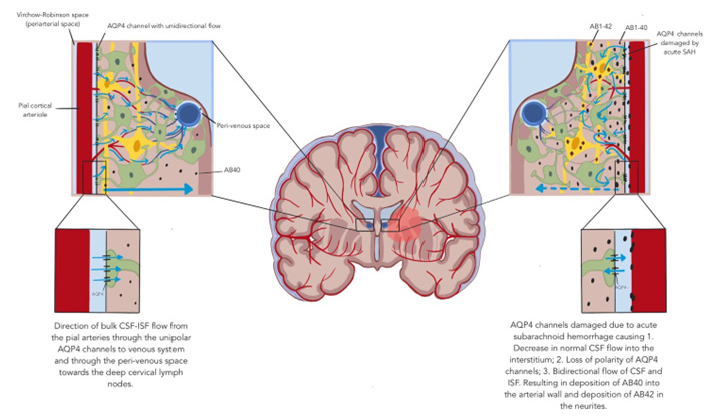
Rowe et al. [34] showed evidence suggesting that Aβ deposition precedes the diagnosis of Alzheimer’s disease by about 15 years. The amyloid PET scan of our patient performed in 2021 showed the presence of diffuse amyloid deposition in both hemispheres. It will be logical to assume that our patient started Aβ deposition in her brain before she developed her acute subarachnoid hemorrhage. Her amyloid PET scan did show some asymmetry with more deposition in her right hemisphere, another evidence to suggest the impaired glymphatic function may have accelerated the Aβ deposition in the cerebral neurites.
Conclusion
The glymphatic system involving the CSF, brain ISF, and the astrocytic foot processes lined with AQP4 channels should be considered as an important waste clearance system of our brain. It was underrecognized until very recently. It may play an important part in many neurodegenerative disorders including Alzheimer’s disease. Our unique case of the progressive development of amyloid angiopathy and asymmetric hippocampal atrophy following an acute subarachnoid hemorrhage due to rupture of her right posterior communicating artery aneurysm demonstrates strong evidence suggesting dysfunction of the glymphatic clearance system. Future therapeutic approaches may focus more on improving this clearance system for many neurodegenerative disorders.
References
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, et al. (2012) A perivascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4(147): 147ra111.
- Iliff J, Simon M (2019) Cross talk proposal: The glymphatic system supports convective exchange of cerebrospinal fluid and brain interstitial fluid that is mediated by perivascular aquaporin 4. J Physiol 597(17): 4417-4419.
- Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7: e40070.
- Davoodi-Bojd E, Ding G, Zhang L, Li Q, Li L, et al. (2019) Modeling glymphatic system of the brain using MRI. Neuroimage 188: 616-627.
- Taoka T, Naganawa S (2021) Imaging for Central Nervous System (CNS) interstitial fluidopathy: disorders with impaired interstitial fluid dynamics. Jpn J Radiol 39(1): 1-4.
- Watts R, Steinklein JM, Waldman L, Zhou X, Filippi CG (2019) Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. American Journal of Neuroradiology 40(4): 648-651.
- Eide PK, Vatnehol SA, Emblem KE, Ringstad G (2018) Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Scientific Reports 8(1).
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40(12): 2583-2599.
- Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS (2017) Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6: e27679.
- Smith AJ, Verkman AS (2018) The “glymphatic” mechanism for solute clearance in Alzheimer's disease: game changer or unproven speculation? FASEB J 32(2): 543-551.
- Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SA, et al. (2018) Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI insight 3(13): e121537.
- Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256(5054): 184-185.
- Tanzi RE (2005) The synaptic Aβ hypothesis of Alzheimer disease. Nat Neurosci 8(8): 977-979.
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, et al. (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38(9): 1205-1235.
- Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, et al. (2006) Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain 129(11): 2805-2807.
- Yamada M (2015) Cerebral amyloid angiopathy: emerging concepts. J Stroke 17(1): 17-30.
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, et at. (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Act Pharmacol Sin 38(9):1205-1235.
- Plog BA, Nedergaard M (2018) The glymphatic system in central nervous system health and disease: past, present and future. Annu Rev Pathol 13: 379-394.
- Taoka T, Naganawa S (2020) Neurofluid dynamics and the glymphatic system: a neuroimaging perspective. Korean J Radiol 21(11): 1199-1209.
- Orešković D, Klarica M (2010) The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain res rev 64(2): 241-262.
- Nakada T, Kwee IL (2019) Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 25(2): 155-166.
- Watts R, Steinklein JM, Waldman L, Zhou X, Filippi CG (2019) Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. AJNR Am J Neuroradiol 40(4): 648-651.
- Eide PK, Vatnehol SA, Emblem KE, Ringstad G (2018) Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Scientific Reports 8(1).
- Iliff JJ, Nedergaard M (2013) Is there a cerebral lymphatic system? Stroke 44(6): S93-S95.
- Kaur J, Davoodi-Bojd E, Fahmy LM, Zhang L, Ding G, et al. (2020) Magnetic resonance imaging and modeling of the glymphatic system. Diagnostics 10(6): 344.
- Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, et al. (2013) Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. Journal of Translational Medicine 11(1): 1-9.
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, et al. (2014) Impairment of perivascular clearance pathways in the aging brain. Annals of neurology 76(6): 845-861.
- Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, et al. (2020) Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends in Molecular Medicine 26(3): 285-295.
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, et al. (2013) Sleep drives metabolite clearance from the adult brain. Science 342(6156): 373-377.
- Hauglund NL, Pavan C, Nedergaard M (2020) Cleaning the sleeping brain–the potential restorative function of the glymphatic system. Current Opinion in Physiology 15: 1-6.
- Biffi A, Greenberg SM (2011) Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 7(1): 1-9.
- Greenberg SM, Charidimou A (2018) Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke 49(2): 491-497.
- Brenowitz WD, Nelson PT, Besser LM, Heller KB, Kukull WA (2015) Cerebral amyloid angiopathy and its co-occurrence with Alzheimer's disease and other cerebrovascular neuropathologic changes. Neurobiol Aging 36(10): 2702-2708.
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, et al. (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31(8): 1275-1283.
© 2022 Forshing Lui. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






